Smartphone-Based DNA Diagnostics Detects Malaria
By LabMedica International staff writers
Posted on 16 Aug 2021
There remains a substantial burden from infectious disease in low-resource rural communities, not least as a consequence of malaria. In infectious disease diagnosis, results need to be communicated rapidly to healthcare professionals once testing has been completed so that care pathways can be implemented.Posted on 16 Aug 2021
Diagnostic testing continues to underpin control and prevention strategies, primarily through the use of rapid, point-of-care, lateral flow immunoassays, which are affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and deliverable devices. This represents a particular challenge when testing in remote, low-resource rural communities, in which such diseases often create the largest burden.
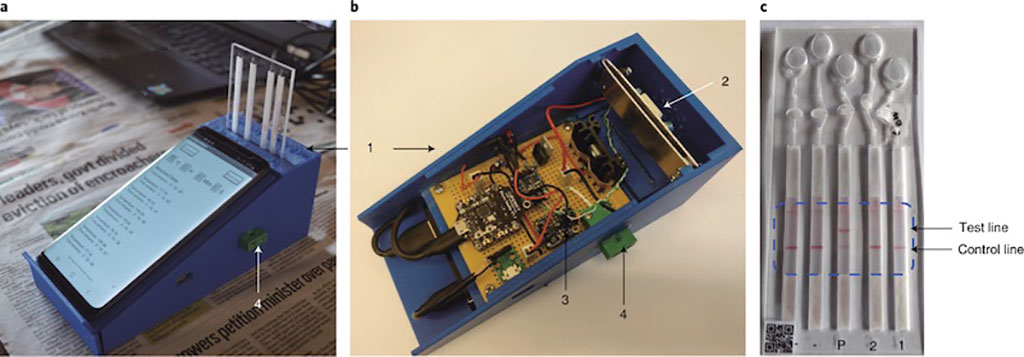
Image: Smartphone-based DNA diagnostics for malaria detection using deep learning for local decision support and blockchain technology for security (Photo courtesy of University of Glasgow)
Bioengineers at the University of Glasgow (Glasgow, UK) and their colleagues developed a smartphone-based end-to-end platform for multiplexed DNA diagnosis of malaria. The diagnostic platform comprises both hardware and software. The hardware includes a three-dimensional (3D) printed mobile heater for loop-mediated isothermal amplification (LAMP)-based diagnostics as well as a mobile phone and a low-cost disposable sensor cartridge, while the software includes an Arduino program, an Android app and a Hyperledger blockchain network.
The team field tested the platform on blood samples collected from 40 school children from Uganda, and compared their results with the gold-standard PCR assay. The team also used malaria rapid immunodiagnostic tests (RDT) for comparison.
The scientists reported that of the 28 tests that were correctly assigned and valid, 16 were true positives (positive for the manually recorded test, the blockchain records and real-time PCR), six were true negatives, three were false negatives and three were false positives (with respect to the gold standard). The blockchain implementation ensured the security of transactions, opening up the possibility for integration into surveillance databases, while maintaining the required safety around data privacy.
The authors concluded that the smartphone-based end-to-end platform they had developed for multiplexed DNA-based lateral flow diagnostic assays that can be used in remote, low-resource settings. Their decision support tool provides automated detection of the results and their analysis, supporting human expertise, and transactions involved in data handling are secured, trusted and endorsed using blockchain technology. The study was published on August 2, 2021 in the journal Nature Electronics.
Related Links:
University of Glasgow













