Plasma ACE2 Predicts Prognosis of COVID-19 Hospitalized Patients
By LabMedica International staff writers
Posted on 24 Jun 2021
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a devastating global pandemic. The disease caused by SARS-CoV-2 infection has been termed coronavirus disease of 2019 (COVID-19) with clinical manifestations ranging from asymptomatic and subclinical infection to severe hyperinflammatory syndrome and death.Posted on 24 Jun 2021
SARS-CoV-2 binds to the angiotensin converting enzyme 2 (ACE2) receptor enabling entrance into cells through membrane fusion and endocytosis. The ACE2-receptor is distributed in different tissues including vascular endothelial cells, smooth muscle cells, nasal and oral mucosa, enterocytes within the intestines, and is especially abundant in the kidneys and type II alveolar pneumocytes in the lungs.
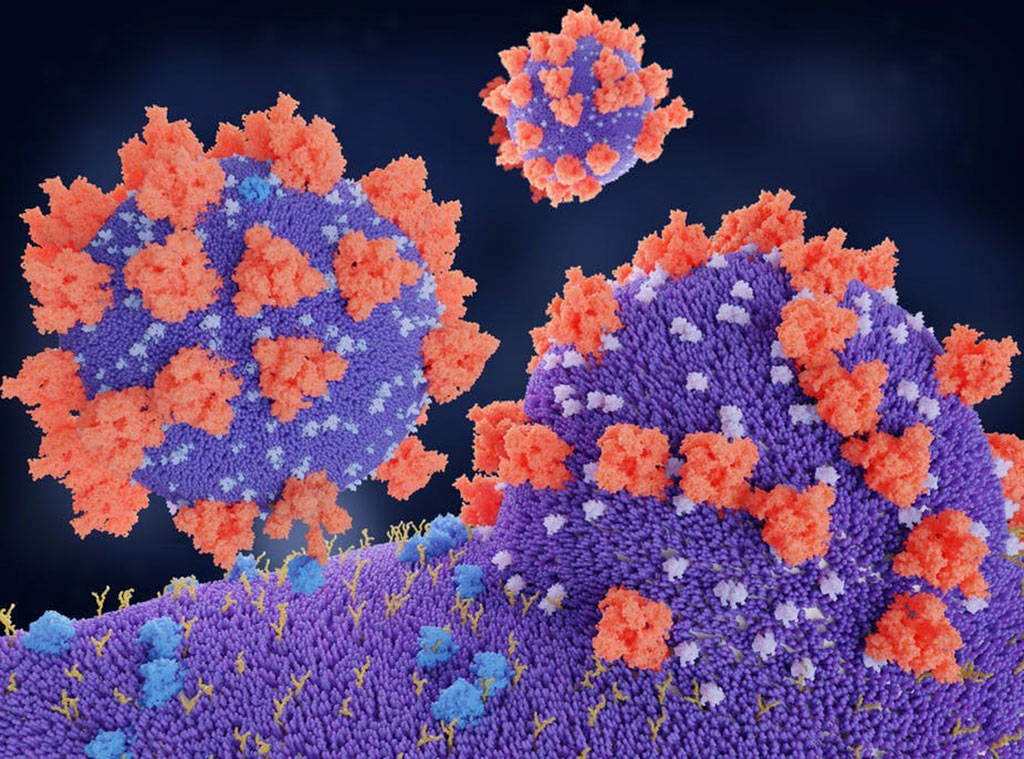
Image: A molecular model of the spike proteins (red) of SARS-CoV-2 binding to the angiotensin-converting enzyme 2 (ACE2) protein, the receptor (blue) which is it’s entry route to the target cell (Photo courtesy of the Juan Gaertner/ Science Photo Library)
An international team of scientists collaborating with those at Aarhus University Hospital (Aarhus, Denmark) analyzed data from a large longitudinal study of 306 COVID-19-positive patients and 78 COVID-19-negative patients (Massachusetts General Hospital Emergency Department COVID-19 Cohort). Subjects included patients 18 years or older, who were in acute respiratory distress with a clinical concern for COVID-19 upon arrival to the Emergency Department. Samples from the patients were analyzed by the Olink Explore 1536 platform (Olink, Uppsala, Sweden), which includes measurement of the ACE2 protein. The Olink platform is based on Proximity Extension Assay (PEA) technology.
The team reported that the analysis demonstrated that high admission plasma ACE2 in patients with COVID-19 was associated with increased maximal illness severity within 28 days (odds ratio [OR] = 1.8). In models correcting for age, body mass index (BMI), hypertension, and pre-existing heart conditions, kidney disease, lung disease, diabetes, and immunosuppressive conditions, significant associations were still observed between plasma ACE2 at admission and maximal illness severity within 28 days.
Further, studies found that plasma ACE2 was also significantly higher in patients with COVID-19 with hypertension compared with patients without hypertension, and in patients with pre-existing heart conditions and kidney disease compared with those without these comorbidities. However, there was no significant difference in plasma ACE2 comparing patients with or without pre-existing lung disease, diabetes, or immunosuppressive conditions. Additionally, elevated plasma ACE2 in COVID-19-positive patients was significantly associated with increasing age, but there was no significant association between plasma ACE2 and BMI.
Tue W. Kragstrup, MD, an associate professor and lead author of the study, said, ““Elevated baseline plasma ACE2 in COVID-19 patients was significantly associated with increased disease severity during the 28-day study period. This indicates that abundant ACE2 production could be involved in increased viral spread and disease burden.”
The authors concluded that the design of the data analysis using the Olink platform does not allow assessment of quantitative differences. However, previous studies have described a positive correlation between plasma ACE2 and ACE1 activity. This is interesting because ACE1 (serum ACE) analysis is a standardized test in most hospital laboratories. Therefore, their study encourages quantitative investigations of both plasma ACE 1 and 2 in COVID-19. The study was published on June 4, 2021 in the journal PLOS ONE.
Related Links:
Aarhus University Hospital
Olink













