Serological Dipstick Assay Developed for Melioidosis
By LabMedica International staff writers
Posted on 05 Aug 2020
The Gram-negative environmental pathogen Burkholderia pseudomallei, causes the severe disease melioidosis. It is highly endemic in southeast Asia and northern Australia, but recent studies suggest that it is also present in many other parts of the world where it is severely underreported. Posted on 05 Aug 2020
The underreporting results from the extremely variable and non-specific clinical manifestations of the disease, lack of clinical recognition, and the global scarcity of good quality laboratories to allow diagnosis from microbiological culture. Early diagnosis of the disease is indispensable for an effective therapy, since B. pseudomallei is intrinsically resistant to many antibiotics used for empirical treatment in endemic areas.
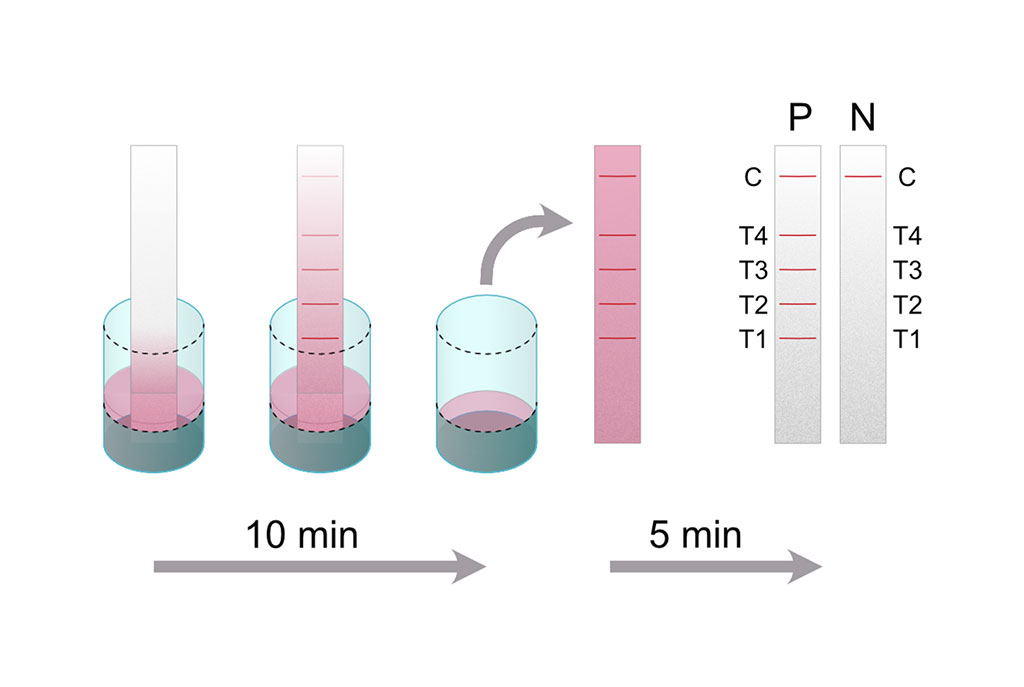
Image: The Melioidosis DS assay principle: A dipstick is placed into a well containing patient serum diluted in dipstick master mix (running buffer, detection antibody and control reagent) (Photo courtesy of Medical University of Graz).
Medical microbiologists at the Medical University of Graz (Graz, Austria) and their international colleagues developed a dipstick assay, which was based on the detection of serum antibodies against four B. pseudomallei specific protein antigens. They evaluated their Melioidosis DS rapid test by using the same set of human serum samples that were previously characterized. The serum collection consisted of 75 sera from culture-confirmed melioidosis patients upon admission and 100 healthy controls. They also tested another 95 samples from Thailand on their dipsticks. These sera were previously classified as 55 false-negative or 40 false-positive on a heme carrier protein 1 (Hcp1) based lateral flow assay. Twenty-eight of the false-positive sera were drawn from healthy individuals and 12 from patients suffering from other kinds of infections.
The investigators reported that their 4-plex dipstick was validated with sera from 75 patients on admission plus control groups, achieving 92% sensitivity and 97% to 100% specificity. They then re-evaluated melioidosis sera with the 4-plex assay that were previously misclassified by the monoplex Hcp1 rapid test. They found that 12/55 (21.8%) false-negative samples were positive in the new dipstick assay. Among those, four sera (7.3%) were Hcp1 positive, whereas eight (14.5%) sera remained Hcp1 negative but gave a positive reaction with their additional antigens.
The authors concluded that the dipstick rapid test represents an inexpensive, standardized and simple diagnostic tool with an improved serodiagnostic performance due to multiplex detection. Each additional band on the test strip makes a false-positive result more unlikely, contributing to its reliability. The study was published on July 13, 2020 in the journal PLOS Neglected Tropical Diseases.
Related Links:
Medical University of Graz











 (3) (1).png)


