Optimized Immunoassays Detect HBV in Oral Fluid Samples
By LabMedica International staff writers
Posted on 05 Sep 2019
It is estimated that there are 257 million chronic hepatitis B (HBV) carriers worldwide, which highlights the substantial health, social, and economic burden of HBV. Screening of infected, cured and vaccinated individuals is necessary to identify the presence of chronically infected reservoirs, immune and susceptible individuals.Posted on 05 Sep 2019
Currently, HBV infection diagnosis is performed using serum and plasma samples collected via venipuncture, which can be invasive, expensive, and potentially painful and arduous for some individuals including the elderly, obese, patients receiving hemodialysis, and drug users. In regions where financial resources are scarce, it would be beneficial to use methods with low cost and biological risk, such as oral fluid samples.
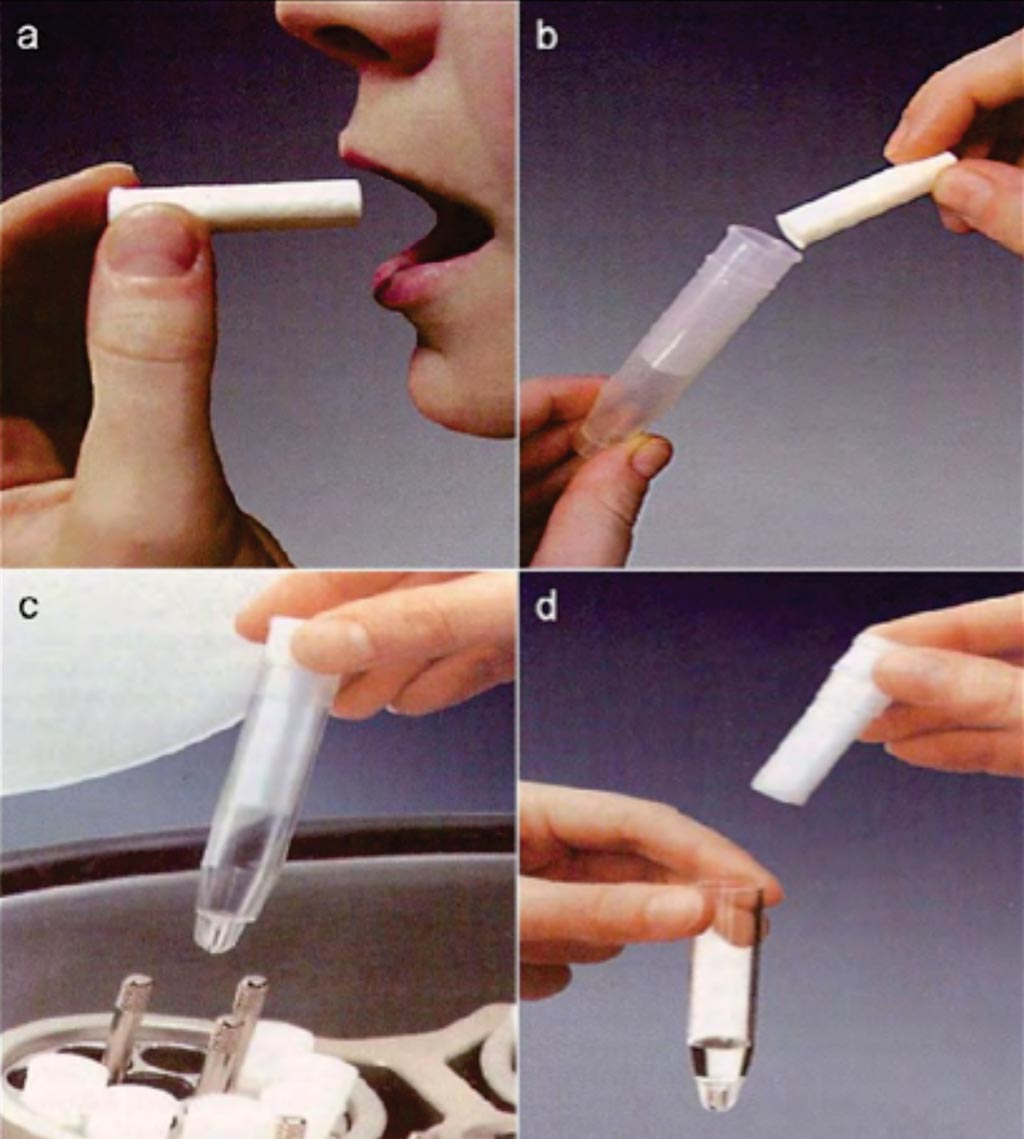
Image: A collection of whole saliva by the Salivette (absorbent) method. (a) Saliva is collected by chewing a cotton wool swab. (b) The swab containing saliva is placed in the tube of Salivette. (c) Centrifugation of the assemblage. (d) Saliva is separated from the swab and is ready for analysis (Photo courtesy of Sarstedt).
Scientists at the Oswaldo Cruz Institute (Rio de Janeiro, Brazil) optimized a commercially available assay for detecting total anti-HBc marker in oral fluid samples and evaluated its utility under real life conditions in different settings for the purposes of prevalence and diagnostic studies. The team collected blood samples, which were centrifuged for serum. Oral fluid was obtained using a commercial Salivette device. All serum samples were submitted to commercial Enzyme immunoassays (EIAs) to detect total anti-HBc antibodies directed against HBV surface antigen (anti-HBs) and HBsAg. All oral fluid samples were also tested with the Diasorin EIA ETI-AB-COREK-PLUS, designed to detect total anti-HBc in serum.
Optimization found the best elution buffer was PBS/BSA 0.5% and the best oral fluid sample volume was 100 µL. The team used these optimized parameters, 1,085 of the samples collected from the 1,296 participants did not detect anti-Hbc; in 211 samples, anti-HBc was detected. The oral fluid sensitivity was 52.6% and specificity was 96%. Sensitivity was higher in patients presenting with an active HBV infection compared with anti-HBc isolate and past infection, 92.7%, 43.2%, and 36.9%, respectively. In addition, sensitivity was higher in participants without active hepatitis C virus infection and also in participants who used injection drugs (85.9%).
The authors concluded that it was possible to optimize a commercial enzyme-linked immunosorbent assay for detecting anti-HBc in oral fluid samples where the highest concordance was found in ambulatory settings and among individuals with active infection. The study was published on July 17, 2019, in the journal BMC Infectious Diseases.
Related Links:
Oswaldo Cruz Institute














