Dual Path Platform Assay Evaluated for Leptospirosis
By LabMedica International staff writers
Posted on 08 Mar 2018
Leptospirosis is an important global cause of acute fever and a leading cause of morbidity among zoonotic diseases and annually, more than one million cases and 50,000 deaths occur worldwide. Approximately 5% to10% of symptomatic patients develop severe manifestations, including multi-system dysfunction and 15% of these may die.Posted on 08 Mar 2018
The gold standards for diagnosing leptospirosis, the microscopic agglutination test (MAT) and hemoculture, have limitations. MAT requires maintenance of reference Leptospira cultures and paired sera for diagnosis, and blood cultures are generally low yield. Early detection of leptospirosis with field-ready diagnostics may improve clinical management and mitigate outbreaks.
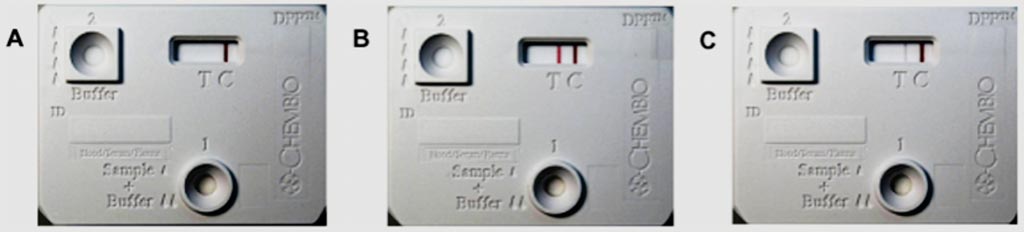
Image: Representative non-reactive (A), strongly reactive (B) and weakly reactive (C) Dual Path Platform (DPP) assay results for leptospirosis (Photo courtesy of the Oswaldo Cruz Foundation).
Scientists from the Oswaldo Cruz Foundation (Salvador, Brazil; www.bahia.fiocruz.br) and their colleagues sequentially enrolled 98 patients hospitalized for acute febrile illnesses, of which they confirmed 32 by leptospirosis reference tests. The patients were from a reference infectious diseases hospital and the specimens were collected from April 18 to October 18, 2012. The study compares the diagnostic accuracy and clinical utility of the point-of-care Dual Path Platform (DPP) using finger stick blood (FSB) against the serum DPP, venous whole blood (VWB) DPP, immunoglobulin-M enzyme-linked immunosorbent assay (IgM-ELISA), and clinical impression.
The DPP (Chembio Diagnostic Systems, Medford, New York, USA; www.chembio.com) utilizes a variation of lateral flow technology, whereby the biological sample and the colorimetric marker are separately delivered on perpendicular nitrocellulose membranes. The team found that DPP sensitivity for classic leptospirosis was 93% by FSB and 96% by VWB. Both POC assays were more sensitive than serum DPP (85%) and serum IgM-ELISA (81%), and similar to clinical impression (96%). The FSB and the VWB DPP detected 40% (2 of 5) and 80% (4 of 5) of the confirmed leptospiral meningitis, respectively, none of which clinicians suspected to be leptospirosis at triage. DPP specificity for classic leptospirosis was 80% by FSB and 75% by VWB.
The authors concluded that the FSB DPP is a rapid, portable alternative to laboratory-based diagnostics for the detection of severe leptospirosis. It expands the diagnostic landscape for effective clinical and outbreak management, and may improve detection of leptospirosis cases presenting with meningitis. The study was published on February 20, 2018, in the journal Public Library of Science Neglected Tropical Diseases.













