New Lyme Disease Tests Offer More Accurate Detection
By LabMedica International staff writers
Posted on 21 Dec 2017
Lyme disease is the most common tick-borne infection in North America and Europe. There are currently over 300,000 cases of Lyme disease annually in the USA alone and the disease is increasing and spreading into new regions.Posted on 21 Dec 2017
Lyme disease frequently, but not always, presents with a bull's-eye rash and when the rash is absent, a laboratory test is needed. New diagnostic methods offer a better chance for more accurate detection of the infection from the Lyme bacteria, the most common tick-borne infection in North America and Europe.
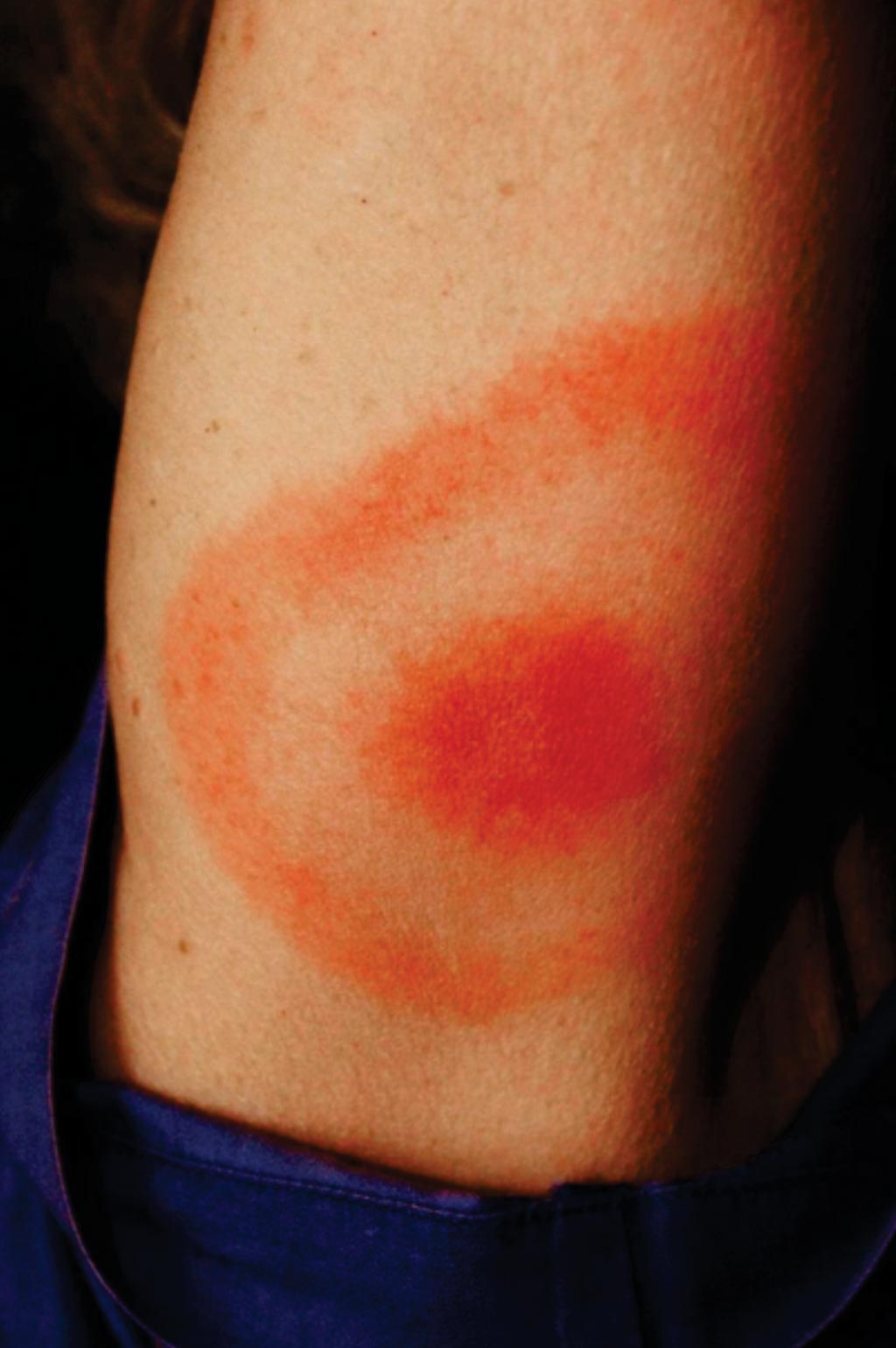
Image: This \"classic\" bullseye rash is also called erythema migrans. A rash caused by Lyme disease does not always look like this and approximately 25% of those infected with Lyme disease may have no rash, hence the need for new diagnostic tests (Photo courtesy of James Gathany/CDC).
Scientists at Rutgers University (New Brunswick, NJ, USA) and their colleagues examined the diagnostic methods employed to detect Lyme disease. A two-tiered testing protocol was established for serodiagnosis in 1994, involving an enzyme immunoassay (EIA) or indirect fluorescence antibody, followed (if reactive) by immunoglobulin M (IgM) and IgG Western immunoblots. These assays were prepared from whole-cell cultured Borrelia burgdorferi, lacking key in vivo expressed antigens and expressing antigens that can bind non-Borrelia antibodies.
Additional drawbacks, particular to the Western immunoblot component, include low sensitivity in early infection, technical complexity, and subjective interpretation when scored by visual examination. Nevertheless, two-tiered testing with immunoblotting remains the benchmark for evaluation of new methods or approaches. Next-generation serologic assays, prepared with recombinant proteins or synthetic peptides, and alternative testing protocols, can now overcome or circumvent many of these past drawbacks. The authors describe next-generation serodiagnostic testing for Lyme disease, focusing on methods that are currently available or near-at-hand.
Steven Schutzer, MD, a physician-scientist and senior author of the study, said, “New tests are at hand that offer more accurate, less ambiguous test results that can yield actionable results in a timely fashion. Improved tests will allow for earlier diagnosis, which should improve patient outcomes. New tests are more exact and are not as susceptible to the same false-positive or false-negative results associated with current tests.” The study was published on December 7, 2017, in the journal Clinical Infectious Diseases.
Related Links:
Rutgers University














