Accurate HIV Self-Test Granted CE Mark
By LabMedica International staff writers
Posted on 31 Oct 2017
Designed for ease-of-use by untrained lay users, the rapid diagnostic self-test consists of a 3rd-generation HIV test-strip in a handheld device that incorporates a sterile safety lancet and unique blood collection and delivery system.Posted on 31 Oct 2017
The Atomo HIV Self Test from Atomo Diagnostics (Sidney, Australia) is the world’s only integrated self-test device, providing wider access to simple, safe, accurate results from a single drop of blood in minutes. This is a 3rd-generation, more sensitive version of its established professional-use rapid diagnostic test Atomo Rapid HIV.
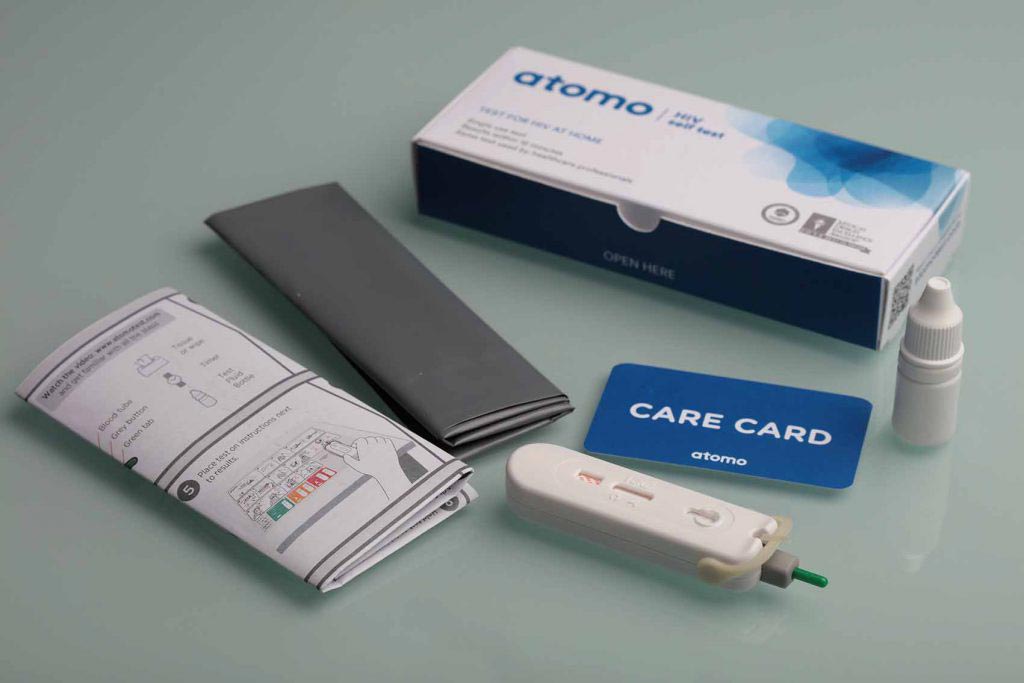
Image: The new Atomo HIV Self Test incorporates a unique blood collection and delivery system to further simplify the test procedure and eliminate user errors common to other test kits (Photo courtesy of Atomo Diagnostics).
The test is a rapid, lateral flow in vitro qualitative immunoassay for detection of antibodies to Human Immunodeficiency Virus Type 1 and Type 2 in human whole blood. It needs only a single drop of blood, obtained from the fingertip using the built-in safety lancet.
When used by untrained users in the field, the Atomo HIV Self Test demonstrated 100% concordance to laboratory results in independent studies, making it the best performing self-test approved to date. Additionally, as a 3rd-generation test, it can detect HIV antibodies earlier than established 2nd-generation competitor tests.
“Self-testing is facilitating greater access to HIV testing for previously hard-to-reach and high-burden groups. Studies have also shown that our test has a high degree of acceptance and take-up amongst the young, which could be central to stemming infection rates and increasing access to treatment,” said John Kelly, CEO, Atomo Diagnostics, “CE Marking means that the Atomo HIV Self Test can now be made available to anyone who wants a safe, convenient, accurate, and private way to find out their HIV status.”
HIV self-testing is increasingly seen as vital, including toward achieving the global health community’s goals stated in the 90-90-90 initiative of the Joint United Nations Programme on HIV/AIDS (UNAIDS). The initiative aims to, by 2020, reach a point where 90% of those living with HIV will know their status, 90% of those individuals will be on antiretroviral therapy (ART), and 90% of individuals on ART will be virologically suppressed. UNAIDS estimated that, as of July 2017, only 70% of people living with HIV knew their status.
“HIV self-testing has the potential to be a game-changer in achieving the UNAIDS ambitious goal of 90% of all HIV positive people knowing their status by 2020,” said Linda-Gail Bekker, President of the International AIDS Society, and member of Atomo’s Clinical Advisory Board.
Atomo HIV Self Test will become available during 2018 via retail, e-commerce, and public health channels.
Related Links:
Atomo Diagnostics














