Molecular Test Detects Chagas in Blood Donors
By LabMedica International staff writers
Posted on 21 Sep 2017
The most prevalent parasitic infection in Latin America causes Chagas’ disease. Diagnosis is based on serologic tests because direct detection of Trypanosoma cruzi is very difficult beyond its brief acute phase.Posted on 21 Sep 2017
In the acute phase, direct detection of parasite is possible; however, this is not reliably sensitive in the chronic phase. For these individuals, diagnosis is based on indirect positive detection by two serologic tests. In 2007, blood centers in the USA began serologically screening blood and organ donors.
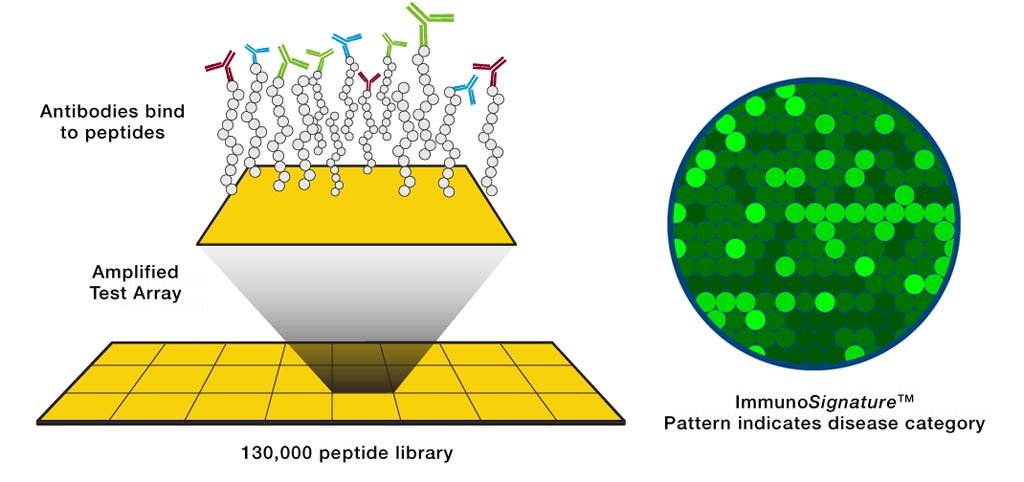
Image: A diagram of the ImmunoSignature Technology (Photo courtesy of HealthTell).
Scientists working at HealthTell, Inc, (Chandler, AZ, USA) collected plasma samples from blood donors in 2015 as samples that were blood panel tested as being serologically-positive for T. cruzi-specific antibodies and corresponding age and gender matched samples that were determined to be T. cruzi antibody-negative. A second cohort of T. cruzi seropositive and seronegative samples was obtained in 2016. These specimens tested negative for all other blood panel diseases. Additional plasma samples that serologically tested positive for hepatitis B virus (HBV), hepatitis C virus (HCV) and West Nile virus (WNV) were obtained in 2016.
The investigators applied HealthTell’s ImmunoSignature Technology (IST) for the detection of T. cruzi-specific antibodies among healthy blood donors. IST is based on capturing the information in an individual’s antibody repertoire by exposing their peripheral blood to a library of more than 100,000 position-addressable, chemically diverse peptides. Assayed microarrays were imaged using an Innopsys 910AL microarray scanner fitted with a 532nm laser and 572nm BP 34 filter.
Initially, samples from two Chagas cohorts declared positive or negative by bank testing were studied. With the first cohort, library-peptides displaying differential binding signals between T. cruzi sero-states were used to train an algorithm. A classifier was fixed and tested against the training-independent second cohort to determine assay performance. Next, samples from a mixed cohort of donors declared positive for Chagas, HBV, HCV or WNV were assayed on the same library. Signals were used to train a single algorithm that distinguished all four disease states. As a binary test, the accuracy of predicting T. cruzi seropositivity by IST was similar, perhaps modestly reduced, relative to conventional enzyme-linked immunosorbent assays.
The authors concluded that the central outcome of their study established IST as a reliable approach for specific determination of T. cruzi seropositivity versus disease-free individuals or those with other diseases. Its potential contribution for monitoring and controlling Chagas lies in IST’s delivery of higher resolution immune-state readouts than obtained with currently used technologies. Despite the complexity of the ligand presentation and large quantitative readouts, performing an IST test is simple, scalable and reproducible. The study was published on September 5, 2017, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
HealthTell














