Antibody-Based ELISA Detects Zika with High Specificity
By LabMedica International staff writers
Posted on 31 Jul 2017
A recently developed human monoclonal antibody-based assay is highly effective in detecting both recent and past Zika virus (ZIKV) infections and in discriminating Zika from other flavivirus infections.Posted on 31 Jul 2017
Investigators at the University of California, Berkeley (USA) and colleagues at the biotechnology company Humabs BioMed (Bellinzona, Switzerland) developed the competitive ELISA based on a human monoclonal antibody specific for the ZIKV nonstructural protein 1 (NS1), which had been developed by Humabs utilizing its proprietary CellClone discovery technology.
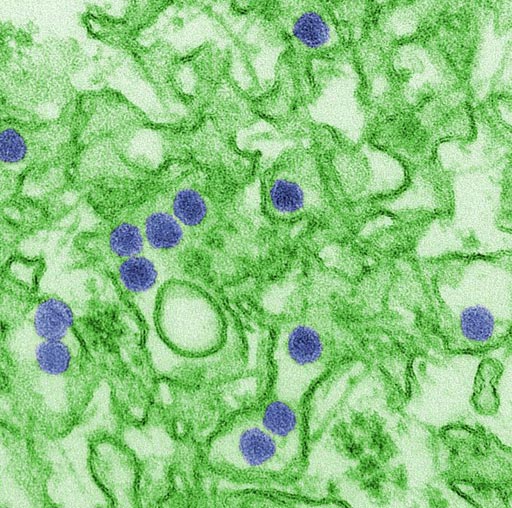
Image: Digitally colorized transmission electron microscopic (TEM) image of Zika virus, which is a member of the family Flaviviridae. Virus particles, here colored red, are 40 nanometers in diameter, with an outer envelope, and an inner dense core (Photo courtesy of Cynthia Goldsmith, CDC).
The assay is in a competitive ELISA format and is based on measuring the presence of plasma or serum antibodies in immune individuals that are able to block the binding of a labelled monoclonal antibody to coated Zika virus NS1. The assay is named NS1 blockade-of-binding (BOB) ELISA and was tested using a large panel of well-characterized clinical samples from travelers and patients living in areas with a high level of exposure to Zika virus and endemic for other flaviviruses.
Of 158 sera/plasma from RT-PCR-confirmed ZIKV infections, 145 (91.8%) yielded greater than 50% inhibition. Of 171 patients with primary or secondary dengue virus infections, 152 (88.9%) scored negative. When the control group was extended to patients infected by other flaviviruses, other viruses, or healthy donors (n = 540), the specificity was 95.9%. Analysis of longitudinal samples from dengue virus-immune and naive ZIKV infections revealed that inhibition was achieved within 10 days following onset of illness and maintained over time.
"The whole world has been in urgent need of a serological method to distinguish dengue virus from Zika virus infections, and this the first to have such high sensitivity and specificity in dengue-endemic regions," said contributing author Dr. Eva Harris, professor of infectious diseases and vaccinology at the University California Berkeley.
"These results support that the antibody-based assay that we have developed is highly effective in detecting both recent and past Zika virus infections and in discriminating Zika from other flavivirus infections," said Davide Corti, senior vice president and CSO of Humabs BioMed. "This novel assay has the potential to become an effective, simple and low-cost solution for Zika surveillance programs, prevalence studies, and clinical intervention trials in flavivirus-endemic areas."
The competitive ELISA for detection of Zika virus was described in the July 17, 2017, online edition of the journal Proceedings of the [U.S.] National Academy of Sciences.
Related Links:
University of California, Berkeley
Humabs BioMed














