Novel Device Reduces Blood Draw Contamination
By LabMedica International staff writers
Posted on 01 Jun 2017
A novel device can significantly reduce contamination of blood cultures, potentially reducing risky overtreatment and unnecessary use of antibiotics for many patients. This approach could also substantially reduce healthcare costs.Posted on 01 Jun 2017
Thousands of patients get their blood drawn every day for blood cultures in order to diagnose serious infections such as sepsis, which can be a deadly condition. A small but significant percentage of the blood cultures are contaminated, due in part to skin fragments containing bacteria that are dislodged during a blood draw.
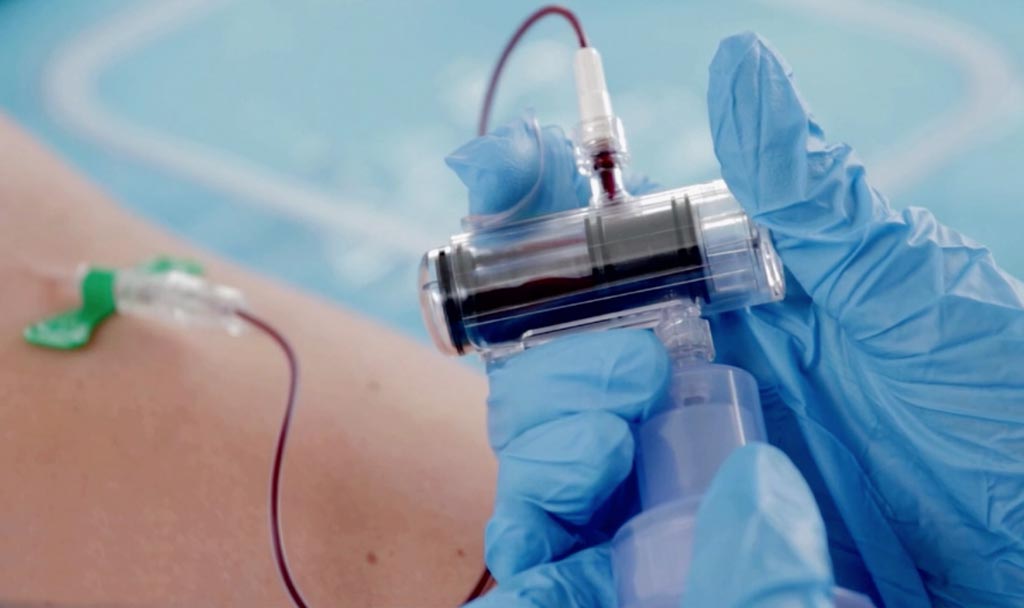
Image: The SteriPath initial specimen diversion device reduces blood culture contamination (Photo courtesy of Magnolia Medical Devices).
Scientists at the University of Nebraska Medical Center conducted a prospective, controlled study with 904 patients and 1,808 blood cultures and compared the standard procedure to an initial specimen diversion device (ISDD) to determine whether blood culture contamination was reduced. The sterile blood collection system diverts and sequesters the first 1.5 to 2 mL of blood, which often carries contaminating skin cells and microbes and this part of the blood is discarded.
The team used the SteriPath initial specimen diversion device and were able to decrease the false positive rate significantly through use of this device, from 1.78% down to 0.2%, which represents an 88% reduction. Contamination rates routinely range from 0.6% to 6% in health care institutions the USA. Costs associated with blood culture contamination per patient case ranged from USD 1,000 in 1998 to USD 8,700 in 2009. A more recent study in the USA observed excess charges of USD 8,720 per contamination event.
Mark Rupp, MD, professor and lead author of the study, said, “A lot of people think this is a minor problem. However, contaminated blood cultures are a big deal. Physicians can be led astray and patients may be harmed by additional tests and unnecessary antimicrobial therapy. What is important about this device is it can greatly limit the blood culture from being contaminated, so physicians are rarely fooled by false-positive results. It gives clinicians confidence that results are accurate.” The study was published on in the journal Clinical Infectious Diseases.