Specificity Improved for Urinary Antigen Testing
By LabMedica International staff writers
Posted on 23 May 2017
The cryptococcal antigen lateral flow assay (CrAg LFA) Immy test is standardized for a fast screening point-of-care test for cryptococcosis, detecting cryptococcal antigen in serum and cerebrospinal fluid (CSF).Posted on 23 May 2017
A delay in laboratory diagnosis is related to sequelae and death and therefore early diagnosis is the key to decrease the high lethality rate due to cryptococcosis. Urine screening would be ideal as a noninvasive approach, but previous studies have shown that fresh urine present false positive results.
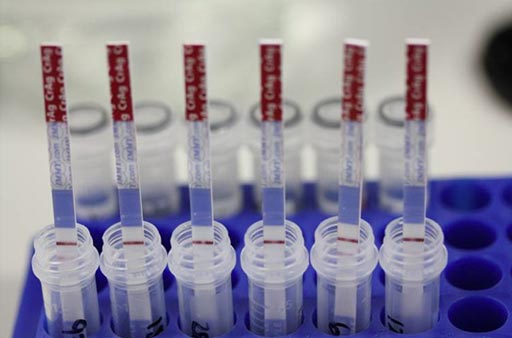
Image: The Cryptococcal Antigen Lateral Flow Assay (CrAg LFA) Immy test (Photo courtesy of Immuno-Mycologics).
Scientists at the National Institute of Infectious Diseases Evandro Chagas performed a prospective cohort study from April 2014 to April 2015 on 77 volunteers: 53 HIV-positive (CD4+ T cell less than 200 cells/mm3) patients, 18 healthy individuals (negative controls), and six HIV-positive patients with active proven cryptococcosis (positive controls). Healthy individuals were included as a negative control and patients presenting proven cryptococcosis as a positive control.
Cryptococcal antigen testing was performed in blood serum and urine from each volunteer using the CrAg LFA Immy test. Each fresh urine sample was tested under two conditions: unheated (untreated) and heated (treated) by five minutes incubation at 100 °C. Clinical specimens such as blood, CSF, and urine were subsequently cultivated to investigate cryptococcal infection. The CrAg LFA in serum samples was considered as the gold standard.
The investigators reported that 24/53 HIV-positive volunteers had a CrAg LFA–positive profile (42.3%) when untreated fresh urine was tested. When heated, only eight samples were positive (15%), presenting 100% of agreement with the positive results obtained from serum samples submitted to CrAg LFA Immy assay. Out of those eight positive patients, five had proven cryptococcosis (positive culture for Cryptococcus neoformans in CSF and/or blood culture) and three patients had cryptococcal antigenemia (negative for Cryptococcus in blood and CSF cultures). The untreated fresh urine has shown 16 false positive results (30.2%). After treatment, those urine samples were negative, as confirmed by negative results in the serum.
The authors concluded that heating of urine prior testing dramatically improved the test’s specificity without compromising the test’s sensitivity. This crucial step has dramatically increased the specificity without compromising test sensitivity, with two additional advantages: no need of enzymatic treatment or sample dilution. The study was published on the journal Public Library of Science Neglected Tropical Diseases.













