Novel Assay Rapidly RSV in Children
By LabMedica International staff writers
Posted on 09 May 2017
A novel rapid assay applies a nicking-enzyme amplification reaction to easily and rapidly detect respiratory syncytial virus (RSV) in children hospitalized with acute respiratory-tract infections.Posted on 09 May 2017
Respiratory syncytial virus is a common respiratory virus that usually causes mild, cold-like symptoms and most people recover in a week or two, but RSV can be serious, especially for infants and older adults. RSV is the most important cause of acute respiratory-tract infection in neonates and young children.
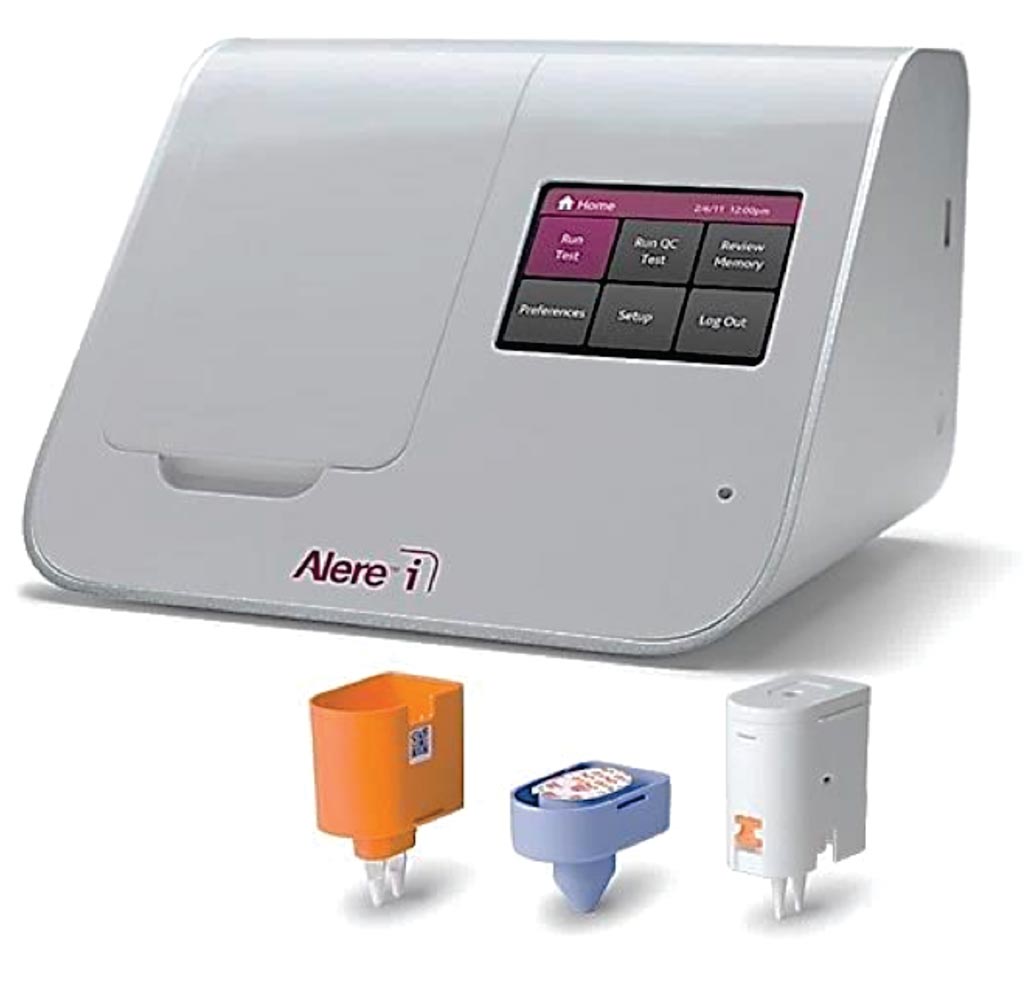
Image: The Alere i RSV assay is designed to easily and rapidly detect respiratory syncytial virus (Photo courtesy of Alere).
Scientists at the University Hospital Heidelberg used the novel assay on 117 frozen nasopharyngeal swab samples obtained from children hospitalized with an acute respiratory-tract infection. The median age of the children whose samples were used in this study was 12 months (range: two weeks to 17.7 years).
The team used the Alere i RSV assay and correctly identified all 49 samples positive for RSV. The assay demonstrated 100% sensitivity (95% confidence interval, CI: 93% to 100%), and 97% specificity (95% CI: 89% to 100%). Testing of 65 negative samples confirmed that 63 samples were true negatives; one false-positive was incorrectly identified, and one sample was invalid. Positive test results were called after a median amplification time of 108 seconds (range: 102 to 234 seconds), with results obtained more quickly in samples with high viral load. The test results compared favorably with real-time reverse transcription polymerase chain reaction (RT-PCR) testing.
Sarah Valerie Schnee, a medical student and lead author of the study, “The total test duration, including three minutes for heating the lysis buffer, was five minutes for positive results and 13 minutes for negative results. The availability of sensitive, rapid RSV tests is critical to optimize care management, minimize unnecessary antibiotic use, and provide targeted infection control.” The study was presented at the 27th European Congress of Clinical Microbiology and Infectious Diseases on held April 22-25, 2017, in Vienna, Austria.












