Molecular Test Improves Diagnosis of Human Babesiosis
By LabMedica International staff writers
Posted on 18 Jan 2017
Babesia species are tick-borne apicomplexan parasites that infect erythrocytes and several species including B. microti, B. duncani, B. venatorum, B. divergens and B. divergens-like organisms can infect humans.Posted on 18 Jan 2017
Babesia infections often are asymptomatic but immunocompromised individuals and even some immunocompetent persons can experience serious, life-threatening manifestations such as splenic rupture, respiratory distress or renal failure. Asymptomatic infected blood donors often are unaware of their infections.
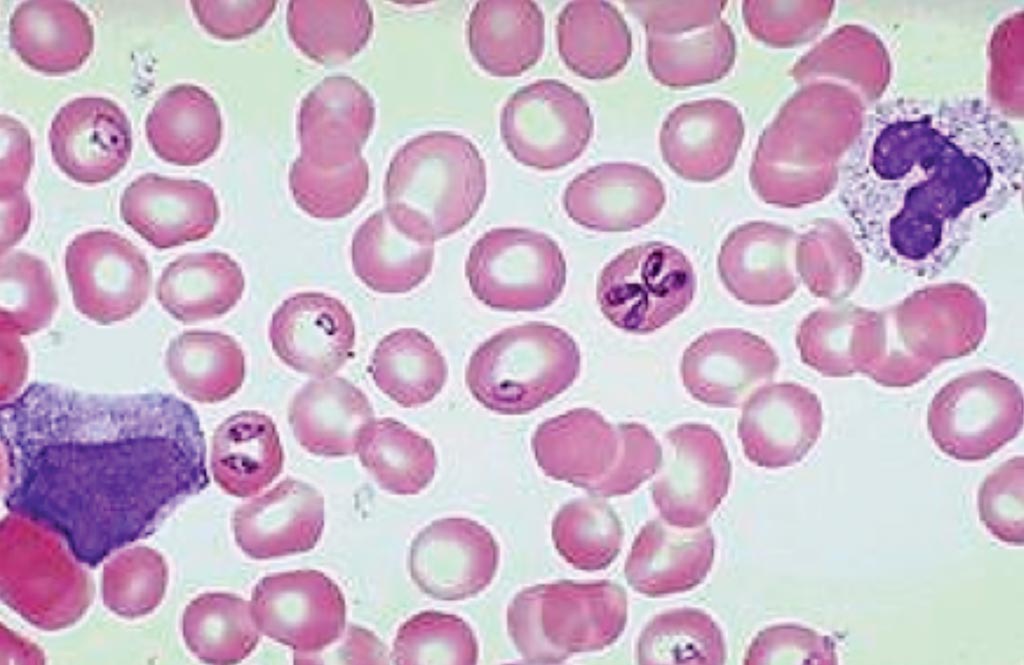
Image: Babesia microti in a thin blood smear stained with Giemsa (Photo courtesy of Spencer S. Eccles Health Sciences Library).
Medical microbiologists and their colleagues at the University of Washington obtained B. microti-containing whole blood samples and negative whole blood samples from de-identified leftover EDTA-anticoagulated clinical samples. The team developed one-step reverse transcription polymerase chain reaction (RT-PCR) for B. microti 18S ribosomal ribonucleic acid (rRNA) as a diagnostic test to take advantage of the biological enrichment of B. microti 18S rRNA as compared to 18S rDNA. This general approach can in future be applied to other Babesia species as well for species specific or pan-Babesia 18S rRNA detection.
Babesia 18S rRNA RT-PCR was performed on an m2000 RealTime System. Nominal densities of B. microti-infected blood samples used for standard curves were measured by counting Giemsa-stained blood smears by microscopy (number of parasites per 2,000 erythrocytes). B. microti-specific primer/probe set was optimized for RT-PCR to generate a highly efficient reaction capable of sub-microscopic B. microti detection.
The assay detected B. microti 18S rRNA in 11 of 11 samples from patients with active or recent B. microti infections. The RT-PCR could detect some samples that may be missed by a PCR-only approach. B. microti 18S rRNA is over 1,000-fold more abundant than its coding genes, making reverse transcription PCR (RT-PCR) much more sensitive than PCR. There was no cross-reactivity against five uninfected blood samples or two high-density Plasmodium falciparum-infected blood samples.
The authors concluded that molecular screening tests for B. microti should be able to detect a single organism in a test volume of ~50–500 μL of whole blood to provide an ultrasensitive limit of detection for use in blood product testing. This limit of detection can likely be achieved using a RT-PCR-based approach and therefore may be useful for screening the blood supply. The study was published on December 5, 2016, in the journal Diagnostic Microbiology and Infectious Disease.














