Rapid Diagnostic Test Performance Assessed for Placental Malaria
By LabMedica International staff writers
Posted on 01 Sep 2016
Women experiencing pregnancy-associated malaria may exhibit normal symptoms of malaria, but may also be asymptomatic or present with more mild symptoms, including a lack of the characteristic fever. This may prevent a woman from seeking treatment despite the danger to herself and her unborn child.Posted on 01 Sep 2016
During pregnancy, a woman faces a much higher risk of contracting malaria and of associated complications. Prevention and treatment of malaria are essential components of prenatal care in areas where the parasite is endemic. The diagnosis of placental malaria is important for program evaluation and clinical care, but is compromised by the suboptimal performance of current diagnostics.
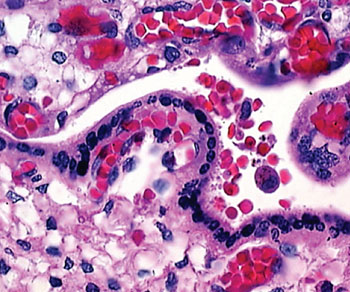
Image: A histopathology of a malaria-infected placenta (Photo courtesy of Dr. Michal Fried).
Scientists at the University of North Carolina (Chapel Hill, NC, USA) and their colleagues used placental and peripheral blood specimens collected from delivering women in Malawi, and compared estimation of the operating characteristics of microscopy, rapid diagnostic test (RDT), polymerase chain reaction, and histopathology using both a traditional contingency table and a latent class analysis (LCA) approach.
The team found that the prevalence of placental malaria by histopathology was 13.8%, but concordance between tests was generally poor. Relative to histopathology, RDT sensitivity was 79.5% in peripheral and 66.2% in placental blood; using LCA, RDT sensitivities increased to 93.7% and 80.2%, respectively. The scientists concluded that their results, if replicated in other cohorts, indicate that RDT testing of peripheral or placental blood may be suitable approaches to detect placental malaria for surveillance programs, including areas where intermittent preventive therapy in pregnancy is not used. The study was published on August 15, 2016, in the American Journal of Tropical Medicine and Hygiene.
Related Links:
University of North Carolina














