Novel Test May Enable Quick Reliable Detection of Sepsis
By LabMedica International staff writers
Posted on 20 Jun 2016
Researchers have developed a rapid specific diagnostic assay that could help physicians decide within an hour whether a patient has a systemic infection. Its potential to detect pathogen materials was demonstrated in animal studies and a prospective human clinical study, whose results also suggested that it could also serve as a companion diagnostic to monitor antibiotic and dialysis-like sepsis therapies.Posted on 20 Jun 2016
The current standard-of-care for detecting blood-borne infections is blood culture, but this takes days, only identifies pathogens in less than 30% of patients with fulminant infections, and is not able to detect toxic fragments of dead pathogens that also drive the exaggerated inflammatory reactions leading to sepsis. Biomarkers that report elevated inflammation are used to monitor treatment of patients with sepsis, but they fail to distinguish inflammation triggered by infectious pathogens from that induced by non-infectious causes (e.g. burns, traumas, surgeries).
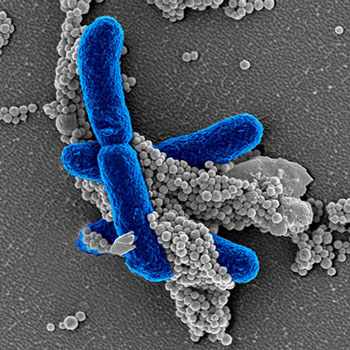
Image: In the new pathogen-detection technology, engineered FcMBL proteins coupled to magnetic beads (grey) specifically bind to carbohydrate molecules on the surface of pathogens, like infectious E. coli (blue) in this electron micrograph, or on fragments of dead pathogens circulating in the bloodstream. After isolation in a magnetic field, the total pathogenic material is quantified with a second FcMBL protein linked to a color-producing enzyme (Photo courtesy of Wyss Institute at Harvard University).
The assay was developed and tested by a research team from Wyss Institute for Biologically Inspired Engineering at Harvard University (Boston, MA, USA) led by Donald Ingber, MD, PhD, Wyss Institute’s founding director, and professor at Harvard Medical School and Boston Children’s Hospital: "Our pathogen detection technology solves both dilemmas: it quickly reports whether infectious pathogens are present in the body, even at early stages of infection before sepsis develops; And it can more specifically identify patients who have excessive inflammation due to systemic infection, rather than other causes," said Prof. Ingber, "This assay could become a real game changer in this clinical area, and it also should lead to more judicious use of antibiotics."
"In a cohort of emergency room patients with suspected sepsis, we saw that the assay picked up infection within an hour in 85% of patients who exhibited clinical symptoms of sepsis, and equally importantly it did not falsely predict infection in healthy subjects or patients with inflammation triggered by other causes, such as trauma. On the other hand, blood cultures that we performed in parallel using the same samples only detected pathogens in 18% of the cases," said Nathan Shapiro, MD, PhD, director, Translational Research, Center for Vascular Biology Research at BIDMC, who worked with the team.
The assay technology is based on magnetic beads to FcMBL, a genetically engineered pathogen-binding protein previously developed by Prof. Ingber and Michael Super, a Wyss senior staff scientist who co-leads the pathogen-detection effort. By recognizing surface carbohydrate molecules, FcMBL binds to pathogens and pathogen-released fragments – pathogen-associated molecular patterns (PAMPs). The team previously established FcMBL as a key component of an advanced dialysis-like, pathogen-extracting therapeutic device, and of a method for fast retrieval of infectious pathogens from complex clinical samples to enable identification and antibody susceptibilities.
"In our latest work, we show that the FcMBL-based pathogen-detecting assay is considerably faster and more accurate than any other available assay for systemic infection. We are currently working to ready it for high-throughput use in clinical and point-of-care situations," said co-lead-author Mark Cartwright, PhD, staff scientist at Wyss.
As a prerequisite to their clinical study, the team had successfully tested the assay in rat and pig models infected with pathogenic E. coli. "The animal models clearly told us that the assay can sensitively trace spikes of PAMPs released during antibiotic therapy, or residual infectious PAMP materials, even when no living bacteria circulate anymore in blood but they remain hidden inside internal organs. Thus, this assay could be an excellent tool for monitoring ongoing infection and responses to antibiotics and dialysis-like therapies for severe infections and sepsis," said Mike Super, PhD.
The findings suggest that this technology, with its rapid handling time, high sensitivity and broad specificity, could provide a real advance for diagnosing infections in clinical microbiology laboratories and point-of-care settings.
The study, by Cartwright M et al, was published online June 12, 2016, in the journal eBioMedicine.
Related Links:
Wyss Institute














