Molecular Methods Compared for Quantitative Detection of Epstein-Barr Virus
By LabMedica International staff writers
Posted on 31 May 2016
Quantitative detection of Epstein-Barr virus by real time polymerase chain reaction (PCR) has become standard of care in the management of immunocompromised patients and is integral to their treatment.Posted on 31 May 2016
The performance characteristics of four real-time assays, three that use different analyte specific reagent (ASRs) and one that uses laboratory-developed reagents, have been compared with one another for the detection of Epstein-Barr virus (EBV) in whole blood.
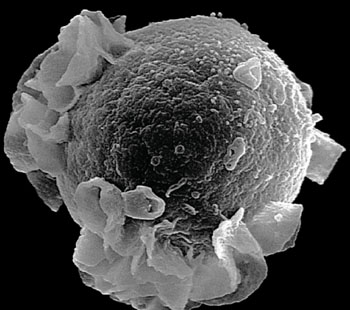
Image: A scanning electron micrograph (SEM) of budding Epstein-Barr virus and a B-cell lymphocyte (Photo courtesy of the Albert Einstein College of Medicine).
Scientists at St. Jude Children's Research Hospital (Memphis, TN, USA) seeded whole blood specimens with EBV, which were used to determine quantitative linearity, analytical measurement range, lower limit of detection, and coefficient of variation (CV) for each assay. Retrospective testing of 198 clinical samples was performed in parallel with all methods; results were compared to determine relative quantitative and qualitative performance.
The ASRs used in the study were produced by Focus Diagnostics (Cypress, CA, USA), Luminex Corporation (Austin, TX, USA), and ELITechGroup (Bothell, WA, USA). The modified laboratory diagnostic test (LDT) was used with the QX100 ddPCR system (Bio-Rad, Hercules, CA, USA) for droplet digital (ddPCR) amplification.
The investigators found that all the assays showed similar performance with no significant difference was found in limit of detection (3.12–3.49 log10 copies/mL). A strong qualitative correlation was seen with all assays that used clinical samples with positive detection rates of 89.5% to 95.8%. Quantitative correlation of clinical samples across assays was also seen in pairwise regression analysis. Normalizing clinical sample results to IU/mL did not alter the quantitative correlation between assays.
The team notes that the Focus ASR reagents can be stored at 4 °C after the first thaw, saving time for future test preparation. The latter assay runs on a 96-well disk in a small volume, and the PCR can be performed in less than an hour, using the 3M Integrated Cycler. However, great attention is needed to load the disk. The other three methods use 96-well plates, and the PCRs were run on an Applied Biosystem sequence detection system 7500, but it could be run on other systems that accept 96-well plates.
The authors concluded that quantitative EBV detection by real-time PCR can be performed over a wide linear dynamic range, using three different commercially available reagents and laboratory-developed methods. EBV was detected with comparable sensitivity and quantitative correlation for all assays. The study was published online on May 22, 2016, in the Journal of Molecular Diagnostics.
Related Links:
St. Jude Children's Research Hospital
Focus Diagnostics
Luminex
ELITechGroup
Bio-Rad