Molecular Assay Compared to Blood Smear for Babesiosis Diagnosis
By LabMedica International staff writers
Posted on 11 Jun 2015
A real-time quantitative polymerase chain reaction (qPCR) has been used to determine the number of DNA copies/mL of blood of a Babesia microti gene in infected patients.Posted on 11 Jun 2015
Although microscopic examination of a peripheral blood smear is the most commonly used diagnostic method to diagnose active Babesia infection in symptomatic patients, polymerase chain reaction (PCR) assays are also being increasingly employed as well.
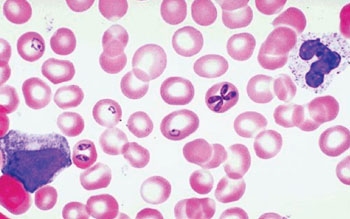
Image: Photomicrograph of a thin blood smear showing different stages of Babesia microti in erythrocytes (Photo courtesy of the University of Utah).
Scientists at New York Medical College (Valhalla, NY, USA) obtained blood samples from 36 patients whose median age was 62.5 years and 75.0% were male with at least one qPCR-positive blood sample were included in this analysis, including 16 with serial blood samples. Blood samples were evaluated by Wright or Giemsa-stained thick and thin blood smears with at least 300 oil immersion fields were examined on a thin blood smear before reporting a negative test result.
Extracted DNA was eluted from the blood samples using a commercial DNA extraction kit, the QIAamp DNA Blood Mini (Qiagen; Valencia, CA, USA) and qPCR was performed on the 7500 Fast Dx Real-Time PCR instrument (Applied Biosystems; Foster City, CA, USA). Of the 36 patients, 32 had one or more positive blood smears for ring forms consistent with B. microti, though not all of the blood samples with positive smears were simultaneously tested by qPCR.
Based on testing of serial blood samples, it was demonstrated that the smear became negative while the qPCR remained positive. A moderate to strong correlation was found between the DNA copy number and the number of infected erythrocytes per milliliter of blood. Based on limited data, the DNA copy number fell by a mean of 4.1% to 12.9% per day on active treatment and by 3.5% to 7.1% per day off therapy.
The authors concluded that the qPCR method is more sensitive than microscopic examination of the blood smear for detection of B. microti infection. This assay should enable an assessment of the rate of parasite clearance over time even in patients who have become smear negative. Quantitative test results for parasite clearance may be useful in evaluating the relative efficacy of various antiparasitic drug regimens and other therapeutic modalities. The study was published in the June 2015 issue of the journal Diagnostic Microbiology and Infectious Disease.
Related Links:
New York Medical College
Qiagen
Applied Biosystems