A New Rapid Chlamydia Test Suitable for Clinical Labs and POC Use
By LabMedica International staff writers
Posted on 25 Dec 2013
A new rapid assay for Chlamydia trachomatis based on a state-of-the-art nucleic acid amplification technique detects as few as 5 to 12 pathogens per test within 20 minutes directly from urine samples without DNA purification.Posted on 25 Dec 2013
Chlamydia trachomatis is the most common sexually transmitted human pathogen. Infections caused by this organism are a major public health concern because of the potential severe long-term consequences, including an increased risk of ectopic pregnancy, chronic pelvic pain, and infertility. However, approximately two-thirds of women with the infection experience minimal or no symptoms and therefore often go undiagnosed.
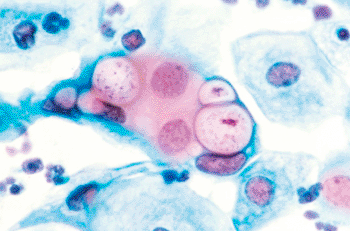
Image: Microgram (500x) of human pap smear showing C. trachomatis in the vacuoles (Photo courtesy of Wikimedia Commons).
To date, several point-of-care tests have been developed for detection of C. trachomatis. Although many of them are fast and specific, they demonstrate only 10% to 40% sensitivity, which is inadequate for large-scale applications.
Investigators at Stockholm University (Sweden) and their colleagues at the University of Tartu (Estonia) have developed an improved test for the detection of C. trachomatis using recombinase polymerase amplification (RPA), a nucleic acid amplification technique that is based on the in vitro synthesis of many copies of DNA or RNA from one original template. The new assay amplifies nucleotide fragments of the C. trachomatis CDS2 gene from urine samples. The assay does not require purification of total DNA from the urine sample. Heating for five minutes at 90 °C is sufficient to release enough of the amplification target to determine whether the pathogen is present. While urine contains polymerase chain reaction (PCR) inhibitors, up to five microliters can be tested without affecting sensitivity of the RPA.
The investigators used the RPA assay to analyze urine samples from 70 patients (51 females and 19 males) attending a sexual health clinic in Estonia. The samples were tested in parallel with RPA and with the Roche (Basel, Switzerland) Cobas Amplicor C. trachomatis assay. Results revealed that 58 samples were negative in both assays (100% specificity). Twelve of the samples tested positive using the Roche assay. Of these, 10 tested positive and two tested negative in the RPA reaction. Based on these results, the clinical sensitivity of the RPA assay can be estimated at 83%.
"The alarmingly poor performance of the available POC tests for C. trachomatis has limited their wider use, and there is a clear requirement for more sensitive and cost-effective diagnostic platforms. Hence, the need for an applicable on-site test that offers reasonably sensitive detection," said senior author Dr. Ülo Langel, professor of molecular biotechnology at the University of Tartu and professor of neurochemistry at Stockholm University. "The [RPA] assay enables highly specific C. trachomatis detection with sensitivity levels significantly improved compared to currently available C. trachomatis POC assays."
The study was published in the January 2014 issue of the Journal of Molecular Diagnostics.
Related Links:
Stockholm University
University of Tartu
Roche














