Clostridium difficile Assay Receives FDA Clearance
By LabMedica International staff writers
Posted on 28 Nov 2013
An assay which detects nucleic acids encoding the toxin A gene and toxin B gene sequences of toxigenic strains of Clostridium difficile has received official clearance.Posted on 28 Nov 2013
The assay, manufactured by IntelligentMDx (Waltham, MA, USA) can detect C. difficile in human liquid or soft stool specimens collected from patients suspected of having Clostridium difficile-associated disease.
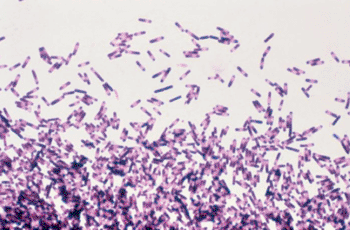
Image: Micrograph of Clostridium difficile made from an impression smear of 72-hour growth on anaerobe blood agar (Photo courtesy of Dr. Gilda Jones).
The assay is called IMDx C. difficile and is designed for use with Abbott m2000 Real Time system (Abbott Molecular; Abbott Park, IL, USA). This is the third test from IMDx to receive US Food and Drug Administration (FDA; Silver Springs, MD, USA) clearance within three months for use on Abbott’s fully automated m2000 system and follows clearances of the IMDx VanR for Abbott m2000 and IMDx Flu A/B and RSV for Abbott m2000 assays.
Analytical reactivity studies demonstrated that the IMDx C. difficile for Abbott m2000 assay is capable of detecting the 31 different toxigenic C. difficile strains that were tested. These strains represent the global diversity of C. difficile strains, including nucleosome assembly protein I (NAP1), and toxin B (tcdB)-variant strains, such as 1470. The IMDx C. difficile for Abbott m2000 assay is part of a broad real-time polymerase chain reaction PCR test menu designed, developed, and manufactured for use on Abbott’s RealTime m2000 system under a multi-year distribution agreement with Abbott.
Effective molecular diagnostic tests for early detection of C. difficile remain critical to help control the spread of infection and the threat of more serious complications. C. difficile is the leading cause of hospital-acquired diarrhea and may cause more severe intestinal conditions such as pseudomembranous colitis. According to the US Centers of Disease Control and Prevention (CDC; Atlanta, GA, USA) C. difficile infection is linked to approximately 14,000 deaths in the USA annually.
Alice Jacobs Nesselrodt, MD, Chairman and CEO of IMDx, said, “FDA clearance of the IMDx C. difficile for Abbott m2000 assay comes at a pivotal time when incidence of C. difficile infection and severity in both hospital and community settings is increasing. Detection of a wide variety of C. difficile strains, including hypervirulent strains, by the IMDx C. difficile for Abbott m2000 assay will help ensure that a high number of cases of C. difficile are detected in a timely manner, regardless of the source of infection.”
Related Links:
IntelligentMDx
Abbott Molecular
US Centers of Disease Control and Prevention














