Multiplex Molecular Tests Detect Seven Intestinal Parasites
By LabMedica International staff writers
Posted on 28 Mar 2011
Polymerase chain reaction (PCR) assays for intestinal parasites can be used on fecal DNA samples for enhanced detection of pathogenic organisms.Posted on 28 Mar 2011
A multiplex PCR-based assay for the ova and parasite stool examination is now available and the molecular technology is comparable with microscopy and copro-antigen detection systems.
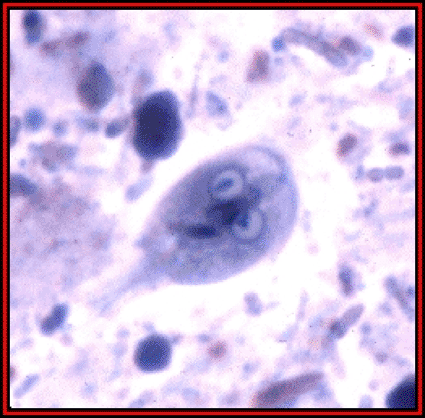
Image: Giardia Lamblia trophozoite (photo courtesy of Raymond Jacobson).
Scientists at the University of Virginia (Charlottesville, VA, USA), adapted several existing real time PCR assays into a high throughput protocol for the major intestinal parasites. Fecal DNA specimens were obtained from 192 preschool-age or younger children from Bangladesh and 190 DNA specimens were obtained from the Leiden University Medical Center (Leiden, Netherlands). Positive control materials were included in this study. The assay involves two multiplex PCR reactions, one with specific primers for the protozoa and one with specific primers for the helminths, after which PCR products are hybridized to beads linked to internal oligonucleotide probes and detected on a Luminex platform (Luminex Corporation, Austin, TX, USA).
The Luminex protozoa assay showed a low limit of detection of 1,000 Giardia lamblia cysts, 100 Cryptosporidium parvum oocysts, and 10 Entamoeba histolytica trophozoites in 200 mg of a stool specimen. The Luminex helminth assay could detect Ancylostoma duodenale, Ascaris lumbricoides, Necator americanus, and Strongyloides stericoralis in very low concentrations. When compared with the parent multiplex real-time PCR assays, this multiplex PCR-bead assay afforded between 83% and 100% sensitivity and specificity on 319 clinical specimens.
The authors concluded that the multiplex PCR-bead protocol provides an alternative high throughput molecular diagnostic platform for specific and sensitive detection of several major intestinal parasites and is a potential alternative to microscopy for equipped laboratories. The study was published in February 2011, in the American Journal of Tropical Medicine and Hygiene.
Related Links:
University of Virginia
Leiden University Medical Center
Luminex Corporation














