Screening for Syphilis Recommended for All Pregnant Women
By LabMedica International staff writers
Posted on 08 Jun 2009
The US Preventive Services Task Force (USPSTF; Rockville, MD; USA) has reaffirmed its 2004 recommendation to screen all pregnant women for syphilis infection. Posted on 08 Jun 2009
The recommended screening for syphilis consists of nontreponemal tests, either the venereal disease research laboratory test or the rapid plasma reagin test. Positive results should be confirmed by a fluorescent treponemal antibody absorbed test or a Treponema pallidum particle agglutination test.
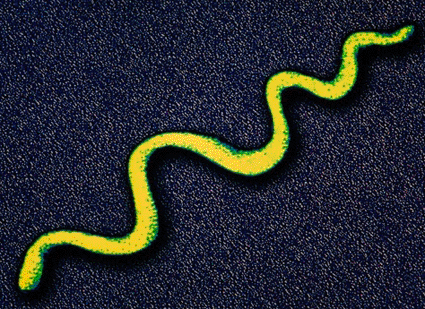
Image: Colored transmission electron micrograph (TEM) showing a Treponema pallidum bacterium, the cause of syphilis in humans (Photo courtesy of Alfred Pasieka / SPL).
Screening should be performed at the first prenatal visit in all pregnant women, as well as in the third trimester and at delivery for women at high risk. Those at high risk include uninsured women, women living in poverty, sex workers, illicit drug users, women diagnosed with sexually transmitted diseases, and those living in communities with high syphilis morbidity.
Failure to treat syphilis during pregnancy may cause stillbirth, neonatal death, bone deformities, and neurologic impairment. Universal screening of pregnant women for syphilis is associated with a lower proportion of infants who have clinical manifestations of syphilis infection, according to observational evidence reviewed by the USPSTF.
The updated recommendation statement and an accompanying review of the underlying evidence are published in the May 19, 2009 issue of the Annals of Internal Medicine.
Related Links:
US Preventive Services Task Force













