Chembio Diagnostics Showcases New DPP Line of Rapid Tests at MEDICA 2019
By LabMedica International staff writers
Posted on 19 Nov 2019
Chembio Diagnostics (Medford, NY, USA) showcased its new DPP line of rapid tests, including DPP Syphilis Screen and Confirm; DPP HIV 1-2 Screen; and, for the global market, DPP Syphilis/HIV combo, the MEDICA 2019 International Trade Fair on November 18-21 in Düsseldorf, Germany. In addition, the company presented its legacy product line of HIV 1-2 SURE CHECK, HIV 1-2 STAT-PAK, HIV 1-2 STAT-PAK Dipstick, as well as its line of unique animal TB diagnostic products.Posted on 19 Nov 2019
The MEDICA is the world's largest medical trade fair for medical technology, electromedical equipment, laboratory equipment, diagnostics and pharmaceuticals. The event drew over 6,000 exhibitors from 70 countries and 120,000 trade visitors from over 170 countries.

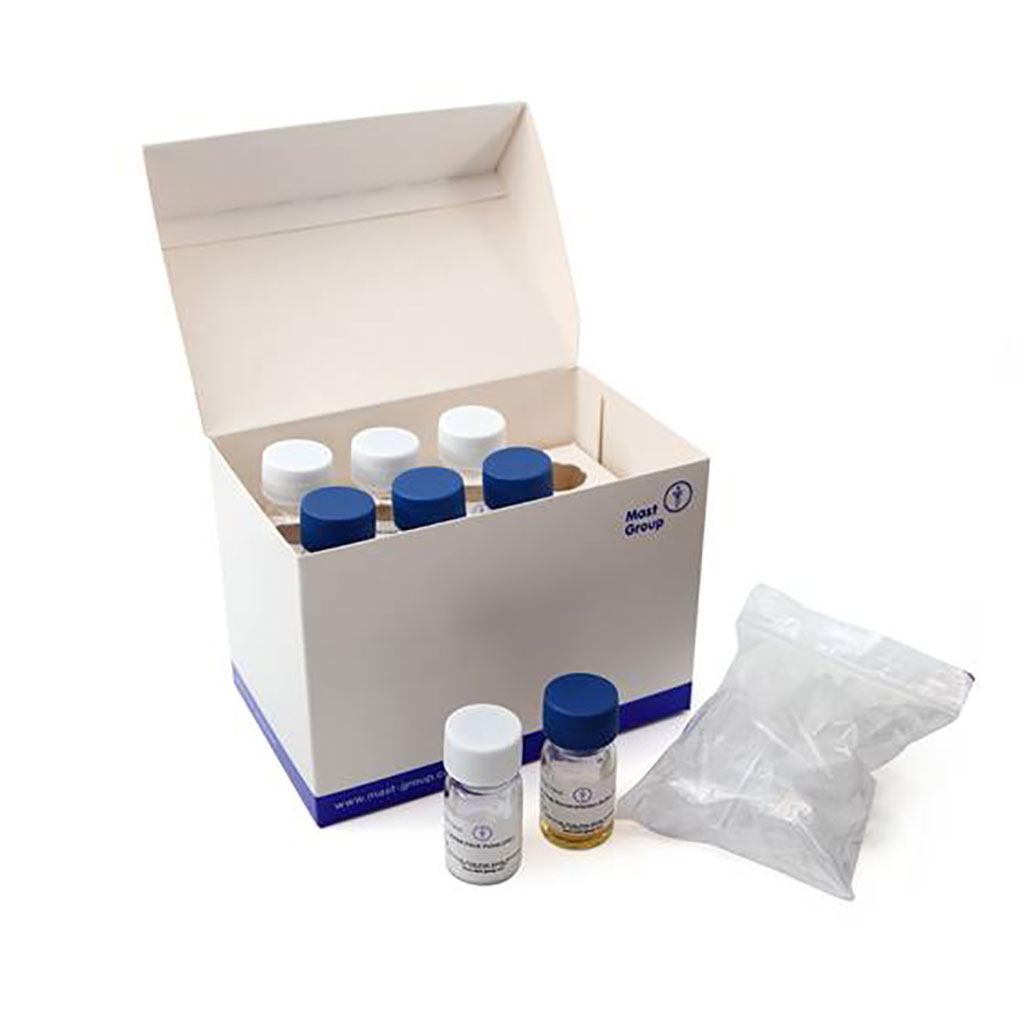
Image: DPP® Syphilis Screen & Confirm Assay (Photo courtesy of Chembio Diagnostics)
Chembio is a leading Point-of-Care (POC) diagnostics company that focuses on detecting and diagnosing infectious diseases. The company’s patented DPP technology platform, which uses a small drop of blood from the fingertip, provides high-quality, cost-effective results in approximately 15 minutes.
At the 2019 edition of MEDICA, Chembio showcased its DPP Syphilis Screen and Confirm Assay (CE Marked), a rapid POC test that simultaneously and separately detects treponemal and nontreponemal antibodies. Since it screens and confirms syphilis infection simultaneously, there is no need for reflex testing and it does not require specialized medical training. It provides results in as little as 15 minutes, enabling physicians to make treatment decisions, while reducing overtreatment rate and cost burden by eliminating repeated visits to healthcare professionals.
Chembio also showcased its DPP HIV 1-2 Screen Assay, a CLIA-waived test for the rapid detection of HIV 1 and HIV 2 antibodies in oral fluid and all blood matrices. It is intended for use as a POC test to aid in the diagnosis of infection with HIV 1 and HIV 2 and is suitable for use in multi-test algorithms that have been established in many countries or as a standalone initial test. For the global market, Chembio showcased its DPP Syphilis/HIV Combo Assay, the first dual HIV 1/2 and Syphilis POC test based on its DPP technology platform. It is a single-use, immunochromatographic, rapid test for the detection of antibodies to Human Immunodeficiency Virus Types 1 and 2 (HIV 1/2) and Treponema pallidum (the causative agent of syphilis) in fingerstick whole blood, venous whole blood, serum, and plasma. A unique feature of this kit is Chembio’s new DPP SampleTainer bottle which contains a pre-measured dilution buffer within a closed vial that also serves as a dropper for performing the assay.
In addition, Chembio showcased its legacy product line, including HIV 1-2 SURE CHECK, a unique, easy-to-use, self-contained, single-use collection and testing device for the rapid, visual detection of antibodies to HIV 1 and HIV 2; HIV 1-2 STAT-PAK, which are easy-to-perform, single-use diagnostic tests for the rapid (15 minutes), visual detection of antibodies to HIV 1 and HIV 2 at POC; and HIV 1-2 STAT-PAK Dipstick, which are easy-to-perform, single-use diagnostic tests for the rapid, visual detection of antibodies to HIV 1 and HIV 2.
Related Links:
Chembio Diagnostics