AI Detects Viable Tumor Cells for Accurate Bone Cancer Prognoses Post Chemotherapy
Posted on 05 Apr 2024
Osteosarcoma, the most common malignant bone tumor, has seen improved survival rates with surgery and chemotherapy for localized cases. Yet, the prognosis for advanced metastatic osteosarcoma remains grim. Traditional post-treatment prognosis methods, based on assessing necrosis or evaluating the proportion of dead tissue within the tumor, suffer from inter-observer variability and might not accurately predict treatment response. Researchers have now developed and validated a machine-learning model capable of accurately evaluating the density of surviving tumor cells in osteosarcoma pathological images, offering a more reliable prognosis prediction.
The model, developed by researchers at Kyushu University (Fukuoka, Japan), uses deep-learning algorithms to identify viable tumor cells within pathological images, matching the assessment skills of expert pathologists. This approach overcomes the limitations of the traditional method for necrosis rate assessment, which calculates the necrotic area without considering individual cell count, leading to inconsistent evaluations across pathologists and inadequate reflection of chemotherapy effects. In phase 1 of the study, the team trained the deep-learning model to detect surviving tumor cells and validated its performance using patient data. The AI model was as proficient in detecting viable tumor cells in pathological images as expert pathologists.
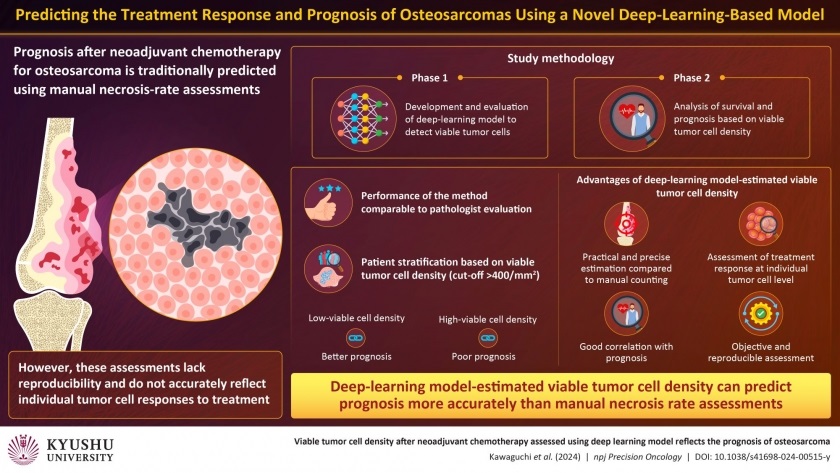
In phase 2, the researchers focused on disease-specific survival and metastasis-free survival. While disease-specific survival tracks the duration after diagnosis or treatment without death directly caused by the disease, metastasis-free survival monitors the time post-treatment without cancer cells spreading to distant body parts. They also examined the correlation between AI-estimated viable tumor cell density and prognosis. The findings revealed that the AI model’s detection performance and precision were comparable to that of the pathologist, accompanied by good reproducibility. The team then divided the patients into groups based on whether the viable tumor cell density was above or below 400/mm2. They found that a higher density correlated with a poorer prognosis, while a lower density indicated a better outcome.
The team found that the necrosis rate was not associated with disease-specific survival or metastasis-free survival. Further analysis of individual cases showed that AI-estimated viable tumor cell density is a more reliable predictor of prognosis than necrosis rate. These findings suggest that by incorporating AI in pathological image analysis, this method enhances detection accuracy, minimizes variability among assessors, and offers prompt evaluations. Estimating viable tumor cell density, which indicates the cells' proliferation potential post-chemotherapy, emerges as a superior indicator of treatment efficacy over traditional necrosis rate assessment. This AI model promises significant advancements in clinical settings after broader validation to facilitate its widespread application.
“This new approach has the potential to enhance the accuracy of prognoses for osteosarcoma patients treated with chemotherapy,” said Dr. Makoto Endo, a lecturer of Orthopedic Surgery at Kyushu University Hospital. “In the future, we intend to actively apply AI to rare diseases such as osteosarcoma, which have seen limited advancements in epidemiology, pathogenesis, and etiology. Despite the passage of decades, particularly in treatment strategies, substantial progress remains elusive. By putting AI to the problem, this might finally change.”
Related Links:
Kyushu University