DNA-Based Blood Test Detects Down Syndrome as Early as the Tenth Week of Pregnancy
By LabMedica International staff writers
Posted on 28 Oct 2014
Down syndrome (Trisomy 21) can now be detected using a noninvasive blood test to evaluate cell-free DNA (cfDNA) found in maternal blood as early as 10 weeks into pregnancy. Posted on 28 Oct 2014
The Ariosa Diagnostics (San Jose, CA, USA) Harmony Non-Invasive Prenatal Test (NIPT) exploits single nucleotide polymorphism (SNP) analysis to precisely quantify cfDNA and determine the fetal DNA contribution in a sample of the mother's blood. Risk assessment for Down syndrome based on fetal DNA measurement is assigned using FORTE, a proprietary algorithm.
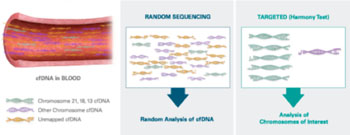
Image: In contrast to tests that use MPSS (massively parallel shotgun sequencing), randomly sequencing all cell-free DNA (cfDNA), the Harmony test focuses on cfDNA from the chromosomes of interest. This unique, directed approach allows deeper analysis and ultimately yields more accurate results (Photo courtesy of Ariosa Diagnostics).
Harmony is cited as being an accurate and reliable test that specifically targets the DNA in chromosomes 21, 18 and 13. While rare, these are the most common trisomies that occur in babies born to women of any age, when no other risk factors are known. While traditional blood tests can miss as many as 15% of Down syndrome cases in pregnant women, Harmony’s DNA-based technology claims to accurately identify more than 99% of cases.
The Harmony test incorporates extensive quality controls. These controls include precise measurement of the amount of the developing baby’s DNA in each sample, to ensure that there is enough DNA present to return a reliable result.
Recently Ariosa announced signing a multi-year supply agreement with Affymetrix (Santa Clara, CA, USA) that will integrate microarrays and instruments produced by Affymetrix into the Ariosa Harmony test framework.
“We are pleased with the Affymetrix partnership as they have been a solid and reliable supplier. Our work on the microarray opens up the possibility to further broaden access to the Harmony test via a kit decentralization strategy,” said Ken Song, CEO of Ariosa. “We are excited about the opportunity to improve prenatal care for women globally.”
“Our array and assay technologies are foundational genomic tools in reproductive health and oncology applications,” said Frank Witney, president and CEO of Affymetrix. “Our partners are providing innovative solutions for unmet needs in these critical areas. We are pleased to work with Ariosa to supply arrays and instrumentation in support of their global business strategy.”
A study published in the June 2014 issue of the journal Fetal Diagnosis and Therapy demonstrated better performance of the Harmony test incorporating Affymetrix microarrays in comparison to a next generation sequencing approach in regard to shorter turn-around time and improved precision in measuring chromosome concentration and fetal fraction of cell-free DNA.
Related Links:
Ariosa Diagnostics
Affymetrix