RNA Biomarker Assay Now Manufactured According to Strict ISO Regulatory Criteria
By LabMedica International staff writers
Posted on 19 Aug 2014
A line of diagnostic kits that measures RNA biomarkers with single-molecule sensitivity is now being manufactured under the stringent requirements of ISO 13485:2003 certification.Posted on 19 Aug 2014
Advanced Cell Diagnostics, Inc. (Hayward, CA, USA), a global technology and market leader in in situ nucleic acid detection for life science research and clinical diagnostics, announced that the production facility for its line of RNAscope assays has received ISO 13485:2003 certification. ISO 13485:2003 certification, issued by the International Organization for Standardization (Geneva, Switzerland), the world's largest developer and publisher of international standards, regulates design, development, production, and commercialization for the implementation of quality management systems and various other technical and operational procedures.
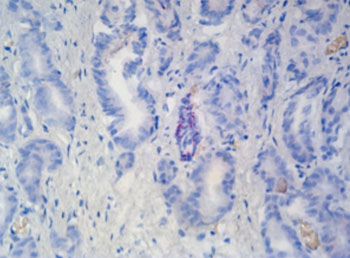
Image: RNAscope detects TP63 mRNA expression in human prostate tissue (Photo courtesy of Advanced Cell Diagnostics).
Radioscope technology is the first automated multiplex RNA in situ hybridization platform capable of detecting and quantifying RNA biomarkers with single-molecule sensitivity. RNA expression levels are highly dynamic and integrate both genetic and epigenetic mechanisms of gene regulation. Thus, RNA expression reflects the state of a biological system. Measuring RNA biomarkers as RNA provides the most direct route for biomarker validation and assay development.
RNAscope employs a proven design strategy similar to fluorescence resonance energy transfer (FRET), in which two independent probes (double Z probes) must hybridize to the target sequence in tandem in order for signal amplification to occur. Because it is highly unlikely that two independent probes will hybridize to a nonspecific target right next to each other, this design concept ensures selective amplification of target-specific signals. For each target RNA species, 20 double Z target probe pairs are designed to specifically hybridize to the target molecule, but not to nontargeted molecules.
"Advanced Cell Diagnostics' vision is to make RNAscope the PCR equivalent for in situ nucleic acid detection and drive adoption of this technology in diagnostic tests," said Dr.Yuling Luo, president and CEO of Advanced Cell Diagnostics. "This ISO certification is the first step in our quality systems road map, which is designed to ensure that we supply products of the highest quality to our customers and provide a seamless path for our partners to translate scientific advances to patient benefits."
Related Links:
Advanced Cell Diagnostics, Inc.
International Organization for Standardization