Inova Diagnostics Receives FDA 510(k) Clearance for Digital Multi-Analyte System Aptiva
By LabMedica International staff writers
Posted on 30 Jun 2021
Inova Diagnostics (San Diego, CA, USA) has received FDA 510(k) clearance for its Aptiva System, a fully automated digital multi-analyte system that represents the next generation of high-throughput processors for the clinical laboratory.Posted on 30 Jun 2021
The company has also been granted FDA 510(k) clearance for its Aptiva Celiac Disease IgA assay. Aptiva addresses many health economic shortcomings in the autoimmune laboratory. Existing systems provide a limited number of analytes that do not reduce the seronegative gap found in many disease states. Beyond the current Celiac assay, Aptiva will include seven additional autoimmune disease states and has over 60 analytes in various stages of advanced development. These additional analytes will help clinicians close the seronegative gap and improve diagnostic confidence.
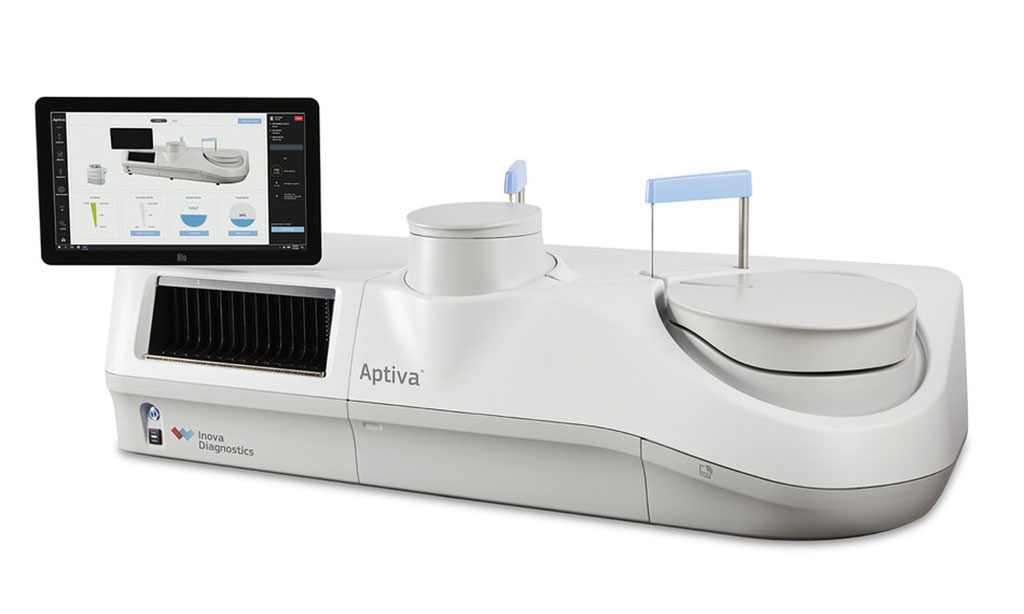
Image: Aptiva utilizes a particle-based multi-analyte technology (PMAT) (Photo courtesy of Inova Diagnostics)
Aptiva also delivers several economic benefits for the laboratory. The 150-sample rack capacity reduces the number of daily interventions and a 6.5-hour consumable walkaway time delivers new levels of workflow efficiencies. Aptiva uses a particle-based multi-analyte technology (PMAT) that processes multiple analytes simultaneously from a patient sample. PMAT enables Aptiva to deliver up to 720 results per hour using a 12-analyte test cartridge and allows the laboratory to complete its workflow in a single shift.
"Aptiva's broad disease and syndromic-based analyte portfolio is a breakthrough that fundamentally enhances the utility of diagnostic testing in the laboratory," said Michael Mahler, PhD., Vice President of Research and Development at Inova. "Aptiva will bring efficiency and reliability to the autoimmune laboratory and provide expanded information to clinicians for management of patients with autoimmune diseases."














