Companion Diagnostic Test Identifies HER2-Ultralow Breast Cancer and Biliary Tract Cancer Patients
Posted on 09 Sep 2025
Breast cancer is the most common cancer in Europe, with more than 564,000 new cases and 145,000 deaths annually. Metastatic breast cancer is rising in younger populations and remains the leading cause of breast cancer-related death. Meanwhile, biliary tract cancer is often diagnosed at advanced stages and carries a poor prognosis with limited treatment options. HER2 is a receptor protein expressed in a variety of cancers and serves as a predictive biomarker to help determine if a patient will respond to HER2-targeted therapy. HER2 interpretation in breast cancer continues to evolve beyond the traditional "positive" or "negative" classifications. Now, a new companion diagnostic test can help expand patient access to personalized treatment for breast cancer and biliary tract cancer.
Roche’s (Basel, Switzerland) VENTANA HER2 (4B5) Rabbit Monoclonal Primary Antibody RxDx assay is intended to identify HR-positive metastatic breast cancer patients with HER2-ultralow status who may be eligible for treatment with ENHERTU. The test can also help identify patients with HER2-positive biliary tract cancer who have an immunohistochemistry score of 3+ and are eligible for treatment with ZIIHERA.
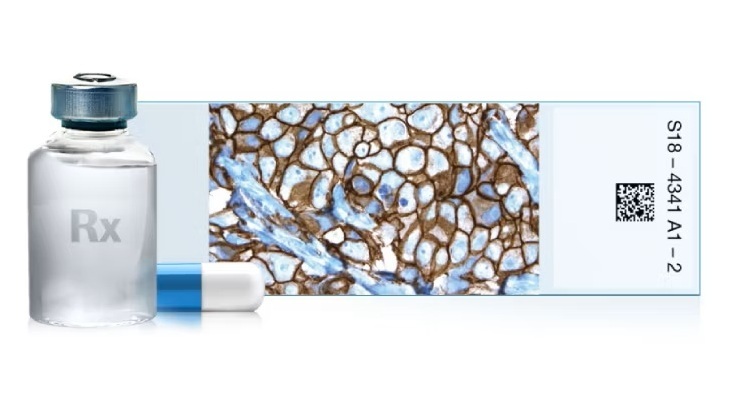
The VENTANA HER2 (4B5) test was used in the DESTINY-Breast06 clinical trial, which showed significant improvement in progression-free survival with ENHERTU compared to standard chemotherapy in patients with HER2-low and HER2-ultralow metastatic breast cancer.
The assay also demonstrated high concordance with HER2 FISH and achieved consistently high proficiency scores across laboratories, ensuring robust validation. The test standardizes immunohistochemistry processes and minimizes variability, ensuring accurate and reliable HER2 assessment.
Roche has received CE IVDR approval for the HER2 (4B5) companion diagnostic test to identify HER2-ultralow breast cancer and biliary tract cancer patients. This expands personalized treatment options for populations with limited therapies. By driving diagnostic certainty, the test empowers laboratories and clinicians to match patients with advanced therapies, ultimately improving care and outcomes across cancer types.
"This is about creating new options for patients facing some of the toughest cancers," said Jill German, Head of Pathology Lab at Roche Diagnostics. "Our understanding of HER2 is rapidly evolving, and this expanded approval ensures our diagnostics are leading the way. We're enabling clinicians to unlock personalized, life-altering treatments for patients who urgently need them."
Related Links:
Roche











 (3) (1).png)


