AI Model Accurately Predicts MSI Tumor and Immune Checkpoint Inhibitor Responsiveness
Posted on 06 Aug 2025
One in three people is expected to develop cancer in their lifetime, and a key factor in patient prognosis is the tumor’s microsatellite status—whether it is stable or shows microsatellite instability-high (MSI-H). MSI-H tumors are associated with better outcomes and are more responsive to immune checkpoint inhibitors (ICIs) compared to microsatellite stable cancers. These tumors, often deficient in mismatch repair proteins, do not necessarily respond well to traditional chemotherapeutics. As a result, experts recommend MSI testing for all newly diagnosed gastric and colorectal cancer patients. While artificial intelligence (AI) has been explored to streamline MSI testing, previous deep learning models have struggled with uncertainty in prediction and failed to deliver insights into ICI responsiveness. Now, a new AI model for accurate MSI prediction as well as immune checkpoint inhibitor responsiveness prediction is expected to help battle gastric and colorectal cancers and further cancer research in general.
A team of researchers from the US and Korea, including from Yonsei University College of Medicine (Seoul, South Korea) and Gangnam Severance Hospital (Seoul, South Korea), has developed MSI-SEER, a deep Gaussian process-based Bayesian model. This AI model analyzes hematoxylin and eosin-stained whole-slide images using a weakly supervised learning framework to predict MSI status in gastric and colorectal cancers. A standout feature of MSI-SEER is its ability to assess its own predictive confidence using Monte Carlo dropout to calculate predictive variance. This is then converted into a Bayesian Confidence Score (BCS), enabling the model to quantify the reliability of each prediction. When uncertainty is high, the system automatically flags those cases for manual review by human pathologists instead of making an autonomous decision. In addition to MSI prediction, the model also incorporates stroma-to-tumor ratio data to improve accuracy in predicting patient responsiveness to ICIs.
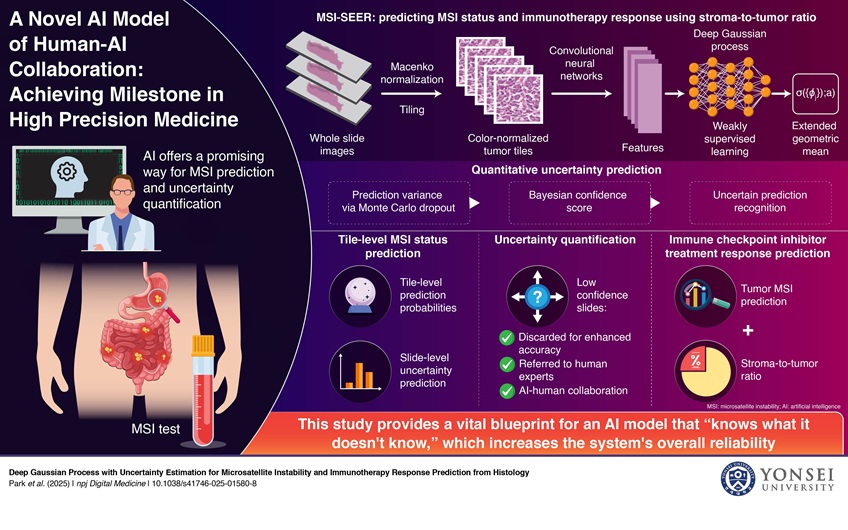
Moreover, MSI-SEER’s tile-level predictions reveal the spatial distribution of MSI-H regions within the tumor microenvironment, offering valuable insights into treatment response. The model was extensively validated using large datasets that included patients from diverse racial backgrounds. The findings, published in npj Digital Medicine, showed that MSI-SEER achieved state-of-the-art performance in MSI prediction by integrating uncertainty estimation. The system also proved highly effective in forecasting ICI responsiveness. These results suggest strong potential for real-world applications, such as in prospective cohort surveillance or as part of post-market (Phase IV) clinical trials. Beyond its immediate predictive power, the innovation points toward a broader future where AI can analyze multi-modal clinical data to support precision oncology. The researchers aim to further refine the model and explore wider clinical deployment.
“We believe our technology already has potential for real-world application as a form of prospective cohort surveillance, or a kind of Phase IV clinical trials,” said Prof. Jae-Ho Cheong, Yonsei University College of Medicine. “The longer-term implication of this study is that it is not about a single specific predictive AI model. Rather, it has a broader implication of how AI algorithm can analyze clinical multi-modal data and create clinically usable models for precision cancer medicine.”
Related Links:
Yonsei University College of Medicine














