Blood Test Could Identify Patients at Risk for Severe Scleroderma
Posted on 16 Apr 2025
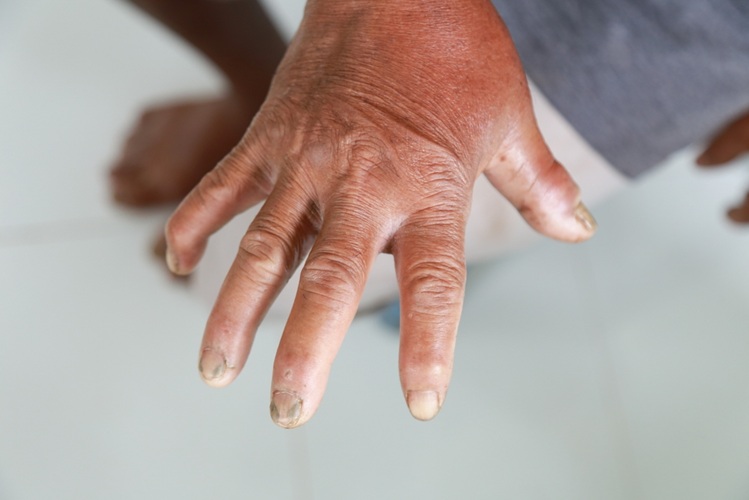
Systemic sclerosis, also known as scleroderma, causes the hardening of the skin and connective tissues. In many cases, the disease can also damage vital organs, including the heart, kidneys, lungs, and gastrointestinal system, potentially leading to life-threatening complications. Among individuals diagnosed with systemic sclerosis, those with diffuse cutaneous systemic sclerosis generally experience a worse prognosis and higher mortality rate compared to those with limited cutaneous systemic sclerosis. Early diagnosis and intervention are crucial to slowing the progression of the disease, but currently, there are no clinical biomarkers to identify patients at higher risk for severe outcomes. Typically, doctors classify patients with suspected systemic sclerosis based on their symptoms: those with skin fibrosis limited to below the elbows and knees are diagnosed with limited cutaneous scleroderma, often experiencing less severe outcomes. On the other hand, patients with diffuse cutaneous systemic sclerosis, where skin fibrosis extends above the knees and elbows and affects other areas of the body, face a more aggressive disease course. Some of these patients may become disabled or develop progressive, debilitating conditions. A new test could now assist in identifying patients at risk for severe scleroderma, as highlighted in a study published in The Lancet Rheumatology.
In this groundbreaking study, researchers led by Yale School of Medicine (YSM, New Haven, CT, USA) demonstrated that type 1 interferons (IFNs)—a group of proteins that play a role in cell signaling—can serve as a blood biomarker for individuals with diffuse cutaneous systemic sclerosis. This discovery marks a significant advancement in early detection of high-risk patients. To find a reliable marker that could help clinicians predict poor outcomes in patients with diffuse cutaneous scleroderma, a team of clinician scientists from 11 academic centers across the United States collaborated to recruit patients with early-stage diffuse cutaneous systemic sclerosis. In 2012, they established the U.S. Prospective Registry of Early Systemic Sclerosis (PRESS), which includes patients who meet specific criteria for early diffuse cutaneous systemic sclerosis. The study involved 110 patients from the PRESS cohort. Simultaneously, researchers in the U.K. recruited a separate cohort of 32 healthy individuals and 72 patients with diffuse cutaneous systemic sclerosis as part of the Stratification for Risk of Progression in Scleroderma (STRIKE) study.
High levels of type 1 IFNs have been associated with worse outcomes in autoimmune diseases such as rheumatoid arthritis and lupus, but measuring blood serum levels of these IFNs is challenging. To address this, the researchers analyzed the concentrations of various molecules that respond to type 1 IFNs and are present in sufficient quantities for measurement, using them as indirect indicators of IFN activity. They discovered that participants in the PRESS cohort with high serum IFN scores tended to experience worse lung function and increased disability, including chronic joint pain, both at the start of the study and during follow-up. In the STRIKE cohort, patients with high IFN serum scores also exhibited poorer lung function, and these differences persisted throughout the study. Across both cohorts, individuals with elevated IFN scores had higher mortality rates than those with lower scores. As scleroderma-related lung disease is the leading cause of death in this patient population, identifying a blood biomarker that could predict patients at greater risk for lung disease is an important breakthrough. The researchers believe that the high serum IFN score could eventually be used to predict which patients, especially in the early stages of their disease, are at risk for developing severe conditions, facilitating more personalized and effective treatment strategies.
“Our results suggest that measuring type I IFN activity is akin to assessing the fuel driving autoimmune processes in systemic sclerosis patients,” said Monique Hinchcliff, MD, MS, at YSM. While additional validation and testing are necessary, “the ability to possibly discriminate between high-risk and low-risk patients with diffuse cutaneous systemic sclerosis using a blood test represents a large step forward for the community.”














