Microfluidic Chip-Based Device to Measure Viral Immunity
Posted on 05 Dec 2024
Each winter, a new variant of influenza emerges, posing a challenge for immunity. People who have previously been infected or vaccinated against the flu may have some level of protection, but how well their immune system "remembers" the previous strain and reacts to the new one can vary. Currently, there is no reliable way to measure this immune “memory.” Now, new research is working to solve this issue with a device designed to assess immune memory in the blood.
When exposed to a virus, white blood cells known as B-cells activate and differentiate. Some of these B-cells become plasma cells that quickly produce antibodies to fight off the infection, while others turn into memory B-cells, remaining dormant until the same or a similar virus reappears. If the virus returns, memory B-cells can swiftly recognize it and produce antibodies to combat it. Presently, measuring circulating antibodies produced by plasma cells is possible, but antibody levels decline over time. It's far more challenging to assess the presence and effectiveness of memory B-cells, especially against new variants of the same virus. In a new project funded by the NIH, researchers from the University of California, Davis (Davis, CA, USA) and Johns Hopkins Bloomberg School of Public Health (Baltimore, MD, USA) have developed a prototype device that measures memory B-cells by testing how well they can adhere to a surface while recognizing the virus under shear flow. This method, called Shear Activated Cell Sorting (SACS), is at the core of their approach.
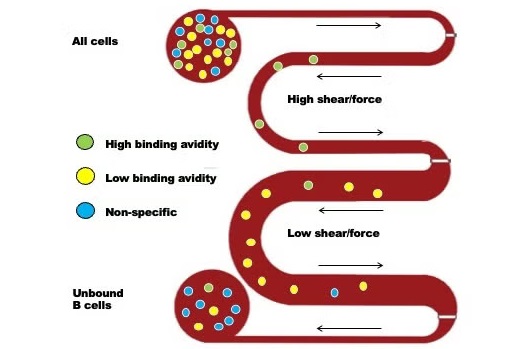
The device works by using a microfluidic chip with tiny channels. The base of the channel is coated with the influenza virus. As white blood cells flow through these channels, memory B-cells that recognize viral proteins (antigens) will attach to the surface. By adjusting the flow rate, researchers can measure how strongly the cells adhere. As the flow rate increases, shear forces are applied to the cells, pulling them off the surface. By tracking how many cells adhere or are washed away at different flow rates, researchers can gauge their binding affinity, i.e., how well the memory cells stick to the virus. This data allows the scientists to compare how well the cells bind to the original virus they were exposed to and a new variant. The ultimate goal of this device is to provide public health labs with a tool to measure immunity to new flu variants in populations, aiding in public health decision-making. Additionally, this technology could be adapted to assess immunity against SARS-CoV-2 and other viruses.
“There’s no way to assess if the immune system is prepared for the next mutant flu virus, so we need a new vaccine every year,” said Steven George, professor of biomedical engineering at UC Davis and co-principal investigator on the grant. “We’re trying to figure out if you have white blood cells that can respond quickly to a new variant.”













