Cancer-Associated T Cell Receptors Evaluated for Malignancy Detection
By LabMedica International staff writers
Posted on 31 Aug 2020
A key goal in oncology is diagnosing cancer early, when it is more treatable. Despite decades of progress, early diagnosis of asymptomatic patients remains a major challenge. Most methods for this involve detecting cancer cells, but a different approach, focused on the body’s immune response.Posted on 31 Aug 2020
The adaptive immune system recognizes tumor antigens at an early stage to eradicate cancer cells. This process is accompanied by systemic proliferation of the tumor antigen–specific T lymphocytes. While detection of asymptomatic early-stage cancers is challenging due to small tumor size and limited somatic alterations, tracking peripheral T cell repertoire changes may provide an attractive solution to cancer diagnosis.
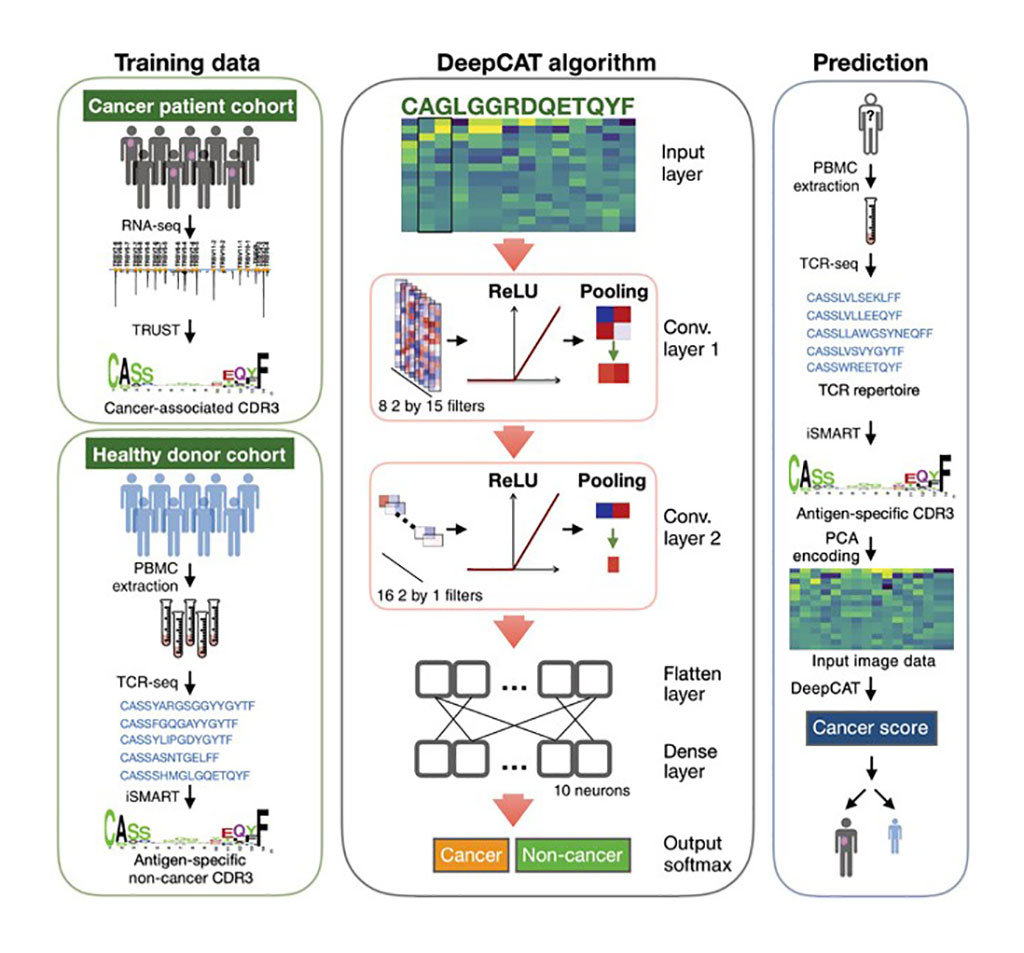
Image: De novo prediction of cancer-associated T cell receptors for noninvasive cancer detection (Photo courtesy of UT Southwestern Medical Center).
Medical scientists at the UT Southwestern Medical Center (Dallas, TX,USA) developed a deep learning method called DeepCAT to enable de novo prediction of cancer-associated T cell receptors (TCRs). They validated DeepCAT using cancer-specific or non-cancer TCRs obtained from multiple major histocompatibility complex I (MHC-I) multimer-sorting studies and demonstrated its prediction power for TCRs specific to cancer antigens. DeepCAT is used by applying a computational method for detecting tumor-infiltrating T cell CDR3 sequences from RNA-sequencing data from thousands of samples from The Cancer Genome Atlas.
The team applied DeepCAT to distinguish over 250 patients with cancer from over 600 healthy individuals using blood TCR sequences and observed high prediction accuracy. DeepCAT was also able to identify cancer TCRs (caTCRs) in blood samples from patients with early-stage kidney, ovarian, pancreatic, or lung cancer. The authors state that the approach does have certain limitations including the inability to determine a cancer's tissue of origin, and they note that inflammatory conditions might affect DeepCAT's performance.
The authors concluded that the cancer score is not intended to replace the current diagnostic methods at this time. Rather, future efforts should be made to explore whether the combined use of the cancer score with existing screening modalities that can improve diagnostic accuracy in patients. This work sets the stage for using the peripheral blood TCR repertoire for noninvasive cancer detection. The study was published on August 19, 2020 in the journal Science Translational Medicine.
Related Links:
UT Southwestern Medical Center














