Blood Test Detects Early Stage Treatable Pancreatic Cancers
By LabMedica International staff writers
Posted on 21 Jan 2015
A recent clinical study has demonstrated that a blood-based diagnostic platform is able to accurately detect 84% of early, surgically-treatable pancreatic cancers with a high 92% specificity. Posted on 21 Jan 2015
The platform technology is able to measure and identify signatures of nucleosomes circulating in the blood. Nucleosomes of disease origin are structurally different in that they have different patterns of histone modification, DNA modifications, DNA methylation or certain adducts from nucleosomes of other cells.
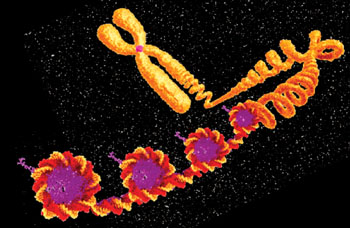
Image: Schematics of the basis of the NuQ assays: A nucleosome is a section of DNA that is wrapped around a core of proteins. DNA is compacted as protein complexes in a “beads on a string” structure (Photo courtesy of VolitionRx Limited).
Scientists at Lund University (Sweden) took blood samples from 25 subjects with stage IIa or stage IIb pancreatic cancer as well as 25 healthy subjects and 10 subjects with other pancreatic diseases including chronic pancreatitis, intraductal papillary mucinous neoplasm (IPMN) which is a precancerous condition which may lead to pancreatic cancer, serous cystadenoma which is a benign tumor and tubular adenoma in papilla vateri, another type of benign tumor.
Analysis of the blood samples demonstrated that a panel of five NuQ assays (VolitionRx Limited; Namur, Belgium) distinguished 21 of the 25 pancreatic cancer cases from healthy subjects, equaling 84% sensitivity, with only two false positive results among the 25 healthy subjects, giving 92% specificity. Furthermore, the same panel of NuQ assays was able to distinguish 19 of the pancreatic cancer cases (76% sensitivity) from all other subjects including healthy subjects and those with other pancreatic diseases with only a single false positive for one healthy subject and two false positives for subjects with other pancreatic diseases, one of which was a subject with precancerous IPMN condition (91% specificity).
Roland Andersson, MD, PhD, professor of surgery, vice-dean, Faculty of Medicine Lund University, said, “In my opinion there are currently no good diagnostic tests for the early detection of pancreatic cancer, so an 84% rate of detection using a simple blood draw is an exciting result, which, in addition to the further ability to differentiate pancreatic cancer from other pancreatic diseases, demonstrates the potential of a test to save many lives and become a significant medical advancement. VolitionRx appears to have a unique platform technology through its technology with circulating nucleosomes. These results are very encouraging and we look forward to confirming the findings in further, larger studies in collaboration with VolitionRx.”
Related Links:
Lund University
VolitionRx Limited