Prototype Rapid Tests Evaluated for Human African Trypanosomiasis
By LabMedica International staff writers
Posted on 08 Jan 2015
The diagnosis of human African trypanosomiasis (HAT) remains a challenge both for active screening, which is critical in control of the disease and in the point-of-care scenario where early and accurate diagnosis is essential. Posted on 08 Jan 2015
The most prevalent species of trypanosome causing HAT, Trypanosoma brucei gambiense, presents a diagnostic challenge and while early diagnosis is essential for effective treatment and also to control transmission, symptoms are nonspecific and parasitological diagnosis is laborious and technically difficult.
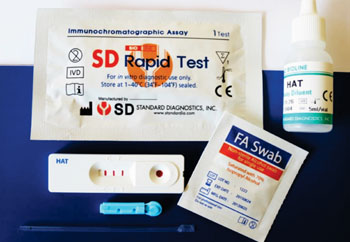
Image: The lateral flow rapid diagnostic test (RDT) for Trypanosoma brucei gambiense, human African trypanosomiasis (HAT) (Photo courtesy of Standard Diagnostics, Inc.).
Scientists at the University of Aberdeen (UK) and their Swiss colleagues carried out a retrospective study using clinical samples of heparinized plasma that were obtained from 250 T. b. gambiense patients and 250 endemic controls. The infection status of patients was confirmed by observation of parasites in the blood, lymphatic system or cerebrospinal fluid, and this provided the reference standard.
Three rapid diagnostic tests (RDT) were evaluated in the study. A registered and commercialized RDT called SD BIOLINE HAT (Standard Diagnostics, Inc., (SD); Yongin, Republic of Korea), that is based on two native variable surface glycoproteins (VSGs). A prototype RDT developed by SD that is based on Baculovirus-expressed recombinant VSG antigens. The third RDT was a prototype RDT that is based on recombinant invariant type-1 trans-membrane domain surface glycoproteins (ISG65) and native VSG (MITat 1.4) antigens developed by BBI Solutions (Cardiff, UK), and called BBI.
The sensitivity and specificity of each RDT was calculated for each reader and each duplicate test. The prototype devices were not inferior in sensitivity or specificity to the SD BIOLINE HAT at the 5% margins, while one of the devices (BBI) had significantly superior sensitivity. Analysis of the performance of individual antigens was used to model new antigen combinations to be explored in development of the next generation of HAT RDTs. The modelling showed that an RDT using two recombinant antigens (rLiTat1.5 and rISG65) would give a performance similar to the best devices in the study, and would also offer the most robust performance under deteriorating field conditions.
The authors concluded that both SD BIOLINE HAT and the prototype devices performed comparably well to one another and also to the published performance range of the card agglutination test for trypanosomiasis in sensitivity and specificity. The performance of individual antigens enabled the team to predict that an all-recombinant antigen RDT can be developed with an accuracy equivalent to SD BIOLINE HAT. Such an RDT would have advantages in simplified manufacture, lower unit cost and assured reproducibility. The study was published on December 18, 2014, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
University of Aberdeen
Standard Diagnostics, Inc.
BBI Solutions














