Novel Biomarker Identified for Two Different Cancers
By LabMedica International staff writers
Posted on 17 Apr 2014
A new biomarker linked to better outcomes for patients with head and neck cancers and non-small-cell lung cancer (NSCLC) has been identified. Posted on 17 Apr 2014
The biomarker could help scientists develop new diagnostics and therapies and help physicians determine the best long-term treatments for patients with these cancers.
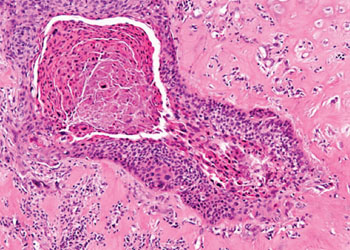
Image: Histopathology of laryngeal squamous cell carcinoma (Photo courtesy of Masaryk University).
Scientists from the University of Pittsburgh (PA, USA) examined tumor samples from 187 patients with non-small-cell lung cancer and 60 patients with head and neck squamous cell carcinomas (HNSCC). The median follow-up for overall survival (OS) and disease-free survival (DFS) for NSCLC patients was 69.0 months and 35.9 months, respectively. The median follow-up for HNSCC patients was 41 months.
The team used antibody-based immunological techniques, cell cultures, immunoprecipitation, immunohistochemistry and in situ quantification. For tumor analysis, tissue microarrays of formalin-fixed and paraffin-embedded tumors and control tissues were used. Each tumor was represented by three distinct cores for NSCLC and two for HNSCC. The nuclear signal intensity was quantified by automated quantitative analysis for NSCLC and Aperio software for HNSCC (Aperio Technologies, Vista, CA, USA).
The investigators found that the expression of a protein called choline phosphate cytidylyltransferase-α CCT-α or CCTα, which acts as an “antigen” that prompts the immune system to produce antibodies against it. CCTα was associated with longer survival rates, including for patients with NSCLC who were treated with surgery alone, without the use of platinum-based chemotherapy drugs and associated toxic side effects.
Laura J. Niedernhofer, MD, PhD, an associate professor and a senior author of the study said, “Based on what we found, a high CCTα expression appears to be indicative of survival, making CCTα a promising biomarker. Our findings suggest that CCTα may, in fact, be more important in determining outcomes in patients with both types of cancer than the already established excision repair cross-complementation group 1 (ERCC1) protein.”
Related Links:
University of Pittsburgh
Aperio Technologies













