Immunoassay Detects Dengue Virus Group Antibodies
By LabMedica International staff writers
Posted on 23 Jan 2014
Dengue virus (DENV) infections are preferentially diagnosed by detection of specific immunoglobulin M (IgM) antibodies, DENV nonstructural protein-1 (NS1) antigen assays or by amplification of viral ribonucleic acid (RNA) in serum samples of the patients. Posted on 23 Jan 2014
Since most antibodies to the four dengue viruses are cross-reacting, a type-specific enzyme linked immunosorbent assay (ELISA) for serum samples, would be beneficial to study the immune response to the circulating viruses in patients, but also in healthy subjects in endemic counties.
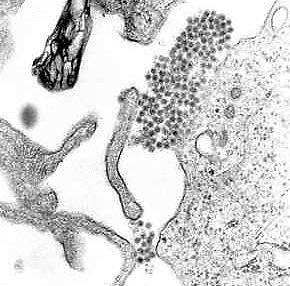
Image: A transmission electron micrograph showing Dengue virus virions (mass of round grey and black shapes appear to the right and few more below (Photo courtesy of the University of South Carolina).
Scientists at the Bernhard Nocht Institute for Tropical Medicine (Hamburg, Germany) collected blood samples over a period of ten years including 64 European tourists with acute dengue fever, whose age range was 20 to 55 years, and with male to female ratio of 1:2. To test the specificity of the DENV immune complex binding (ICB) ELISA serum samples of 88 subjects undergoing routine diagnostics were included.
When serum samples and enzyme- labeled recombinant envelope domain III (EDIII) are incubated together, antigens immune complexes (ICs) are formed, which are simultaneously bound to a solid phase coated with a fragment crystallizable region (Fc)–receptor (CD32). After a single washing procedure, the bound labeled ICs can be determined. To further improve type-specific reactions high concentrations of competing heterologous unlabeled ED III proteins were added to the labeled antigens.
The serum samples of 64 patients with reverse transcriptase polymerase chain reaction (RT-PCR) confirmed primary DENV-1, -2, -3, or -4 infections were tested against four enzyme-labeled recombinant DENV EDIII antigens. The RT-PCR assays were run on a LightCycler 480 System (Roche; Mannheim, Germany). Antibodies to the EDIII antigens were found in 55 patients giving a sensitivity of 86%. A complete agreement between the serotype detected by PCR in early samples and the serotype-specific antibody in later samples was found. Type-specific anti-EDIII antibodies were first detected 9 to 20 days after onset of the disease. In 21% of the samples collected from people in Vietnam, secondary infections with antibodies to two serotypes could be identified.
The authors concludes that the data obtained with the ICB-ELISA show that after primary DENV infection the corresponding type-specific antibodies are detected in almost all samples collected at least two weeks after onset of the disease. The method will be of value to determine the distribution of the various type-specific anti–DENV antibodies in DENV endemic areas. Dengue fever is a highly prevalent arthropod-borne viral disease with 2.5 billion people in tropical or subtropical areas at risk for infection. The clinical picture of dengue may vary considerably from mere fever to severe shock syndrome. The annual number of infections is estimated to several hundred million.
Related Links:
Bernhard Nocht Institute for Tropical Medicine
Roche