Myelodysplastic Syndrome Diagnosed With Peripheral Blood Samples
Posted on 14 Nov 2022
Myelodysplastic syndromes (MDS) encompass a heterogeneous group of clonal bone marrow neoplasms, with a median age at diagnosis of 70 years. MDS are characterized by recurrent cytogenetic and molecular abnormalities, morphologic dysplasia for one or more hematopoietic cell lineage and ineffective hematopoiesis.
Cytomorphological evaluation of bone marrow is the reference standard for the diagnosis of MDS and may be complemented by information obtained from conventional cytogenetic, flow cytometry and molecular profiling analysis. Peripheral blood neutrophil myeloperoxidase expression quantified by flow cytometric analysis has the potential to rule out MDS without requiring invasive bone marrow aspiration.
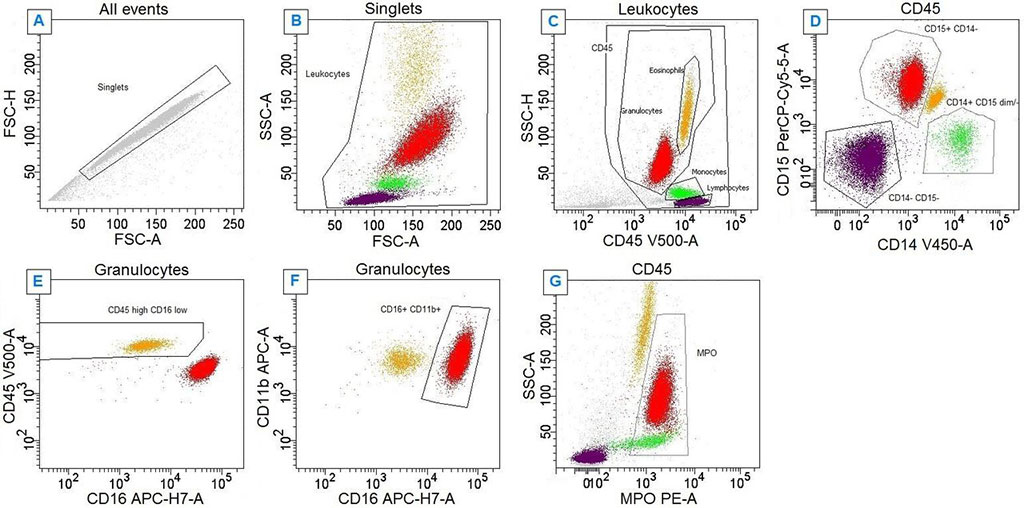
A large team of hematopathologists at the Grenoble Alpes University Hospital (Grenoble, France) and their colleagues are evaluating a cross-sectional diagnostic accuracy study of two index tests by comparison with a reference standard in consecutive unselected adult patients conducted at a single university hospital. The team evaluated a hypothesis that a flow cytometry-based method involving a single-use tube containing lyophilized reagents will provide the accuracy needed to reject a diagnosis of MDS by analysis of peripheral blood neutrophil myeloperoxidase expression, in addition to other hypotheses.
The study examined an approach involving a product called Lyotube Stain 468 (BD Biosciences, San Jose, CA, USA), compared with an approach using a laboratory-developed liquid reagent-based test, in evaluating possible MDS. The Lyotube Stain 468 product includes dried reagents associated with five fluorochromes, while the laboratory-developed test uses liquid reagents associated with the same fluorochromes. With both approaches, an anti-myeloperoxidase antibody is applied to samples, and myeloperoxidase expression from peripheral blood neutrophils is measured. The team used a BD Biosciences three-laser, eight-color BD FACSCanto-II flow cytometer. The primary outcome is the reference diagnosis of MDS or CMML established by bone marrow examination by two independent experienced hematopathologists blinded to the index test results.
The investigators identified the strengths of this study to include the use of an adequate reference approach to MDS diagnosis, limited spectrum bias owing to enrollment of unselected consecutive patients, and a prespecified threshold for evaluation of diagnostic accuracy. Limitations of the study that they identified include the single-center nature of the study setting, in addition to conventional cytogenetic and molecular profiling approaches not being available to all patients in the study. The study was published in the November 2022 issue of the journal BMJ Open.
Related Links:
Grenoble Alpes University Hospital
BD Biosciences













