Personalized CtDNA Analysis Detects Minimal Residual Disease
By LabMedica International staff writers
Posted on 08 Jun 2021
Multiple myeloma (MM), is a type of bone marrow cancer. It is called multiple myeloma as the cancer often affects several areas of the body, such as the spine, skull, pelvis and ribs. Minimal residual disease (MRD) is the name given to small numbers of leukemic cells that remain in the person during treatment, or after treatment when the patient is in remission.Posted on 08 Jun 2021
Despite treatment with high-dose chemotherapy followed by autologous stem cell transplantation (AHCT), MM patients invariably relapse. MRD-negativity post-AHCT has emerged as the most important prognostic marker. Currently, MRD in MM is monitored via bone marrow aspirate sampling. Marrow MRD assays are limited by the spatial heterogeneity of marrow MM localization; extramedullary disease and sampling variability of marrow aspiration.
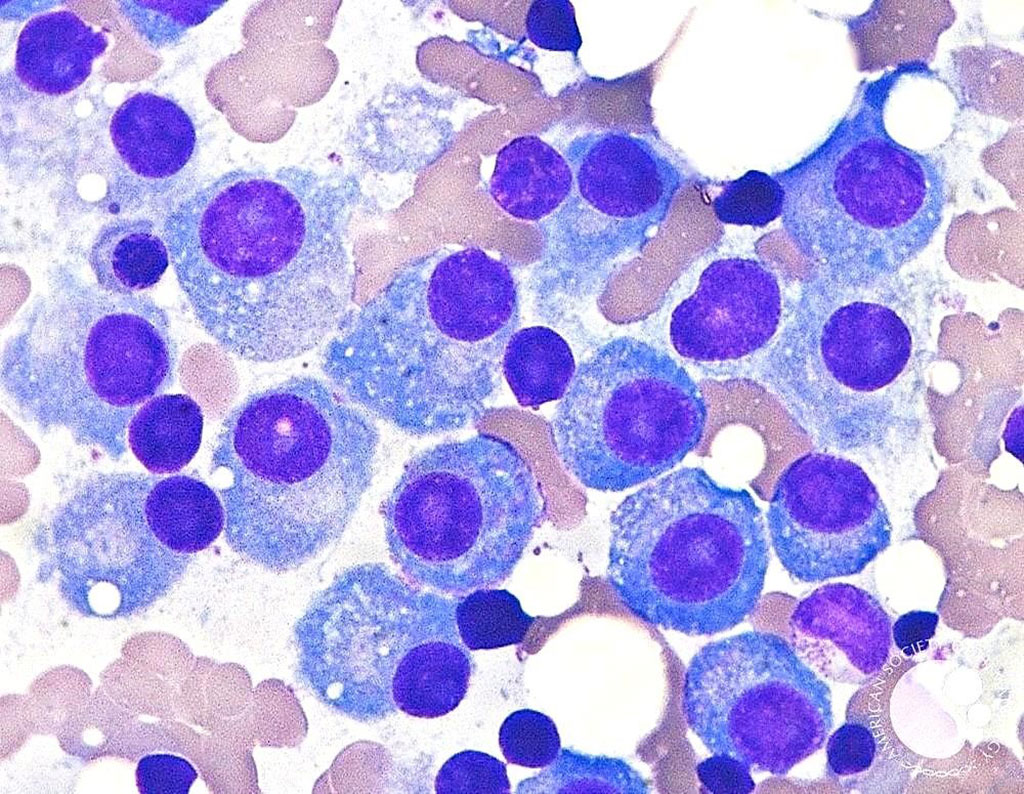
Image: Bone marrow aspirate showing mature plasma cells with eccentric nuclei and abundant basophilic cytoplasm indicative of multiple myeloma (Photo courtesy of Dr. David Israel Garrido, MD et.al.)
Hematologists at the Medical College of Wisconsin (Milwaukee, WI, USA) and their colleagues, analyzed in a retrospective, single-center study, circulating tumor DNA (ctDNA) MRD in blood samples collected from 28 patients with MM after upfront AHCT. A total of 80 plasma time points were available pre and post AHCT with a median follow-up of 92.4 months. Multiparameter flow cytometry (MFC) at 10-4 level was used to assess the MRD from the BM biopsy.
Individual bone marrow aspirates or Formalin-fixed, Paraffin-embedded (FFPE) slides from the time of MM diagnosis and matched normal blood were whole-exome sequenced, and somatic mutations were identified. MRD assessment at three months post-AHCT was performed by ctDNA analysis using a personalized, tumor-informed Signatera bespoke mPCR NGS assay (Natera Inc, San Carlos, CA, USA). The prognostic value of ctDNA was evaluated by correlating MRD status with clinical outcomes.
The scientists reported that ctDNA was detectable in 17/24 (70.8%) of pre-AHCT, 15/28 (53.6%) of ̃three months post-AHCT, and 11/28 (39.2%) of patients during the surveillance phase post-AHCT. Of the 15 ctDNA MRD positive patients, 93.3% experienced relapse on follow-up (hazard ratio: 5.64). Patients negative for ctDNA at three months post-AHCT had significantly superior progression-free survival (PFS) compared to positive (median PFS, 84 months versus 31 months) The positive predictive value (PPV) for relapse among patients positive for ctDNA at three months post-AHCT was 93.3%, and significantly higher than marrow multiparametric flow cytometry (MFC) of 68.4%.
The authors concluded that their study shows the feasibility that a tumor-informed assay on archival blood samples is predictive of relapse post-AHCT. Future prospective studies with real-time marrow next generation sequencing (NGS) and ctDNA samples are needed to define the role of ctDNA in MM and its prognostic significance. The study was presented at the virtual 2021 ASCO Annual Meeting held June 4-8, 2021.
Related Links:
Medical College of Wisconsin
Natera Inc













