New Definition for Plasma Cell Leukemia Proposed
By LabMedica International staff writers
Posted on 09 Jan 2019
Plasma cell leukemia (PCL) is an uncommon but aggressive malignancy that accounts for 1% to 2% of all plasma cell dyscrasias. PCL has been defined as the presence of ≥ 20% circulating plasma cells (CPCs) on conventional white blood cell differential count and an absolute plasma cell count of ≥ 2 × 109/L in the peripheral blood.Posted on 09 Jan 2019
This definition includes both primary PCL (pPCL), arising de novo, in the absence of antecedent multiple myeloma (MM), and secondary PCL (sPCL), which describes leukemic transformation of MM. PCL is clinically and biologically distinct from MM with a younger age at diagnosis, higher propensity to show visceral and extramedullary involvement, and unique biologic, immunophenotypic and cytogenetic characteristics.
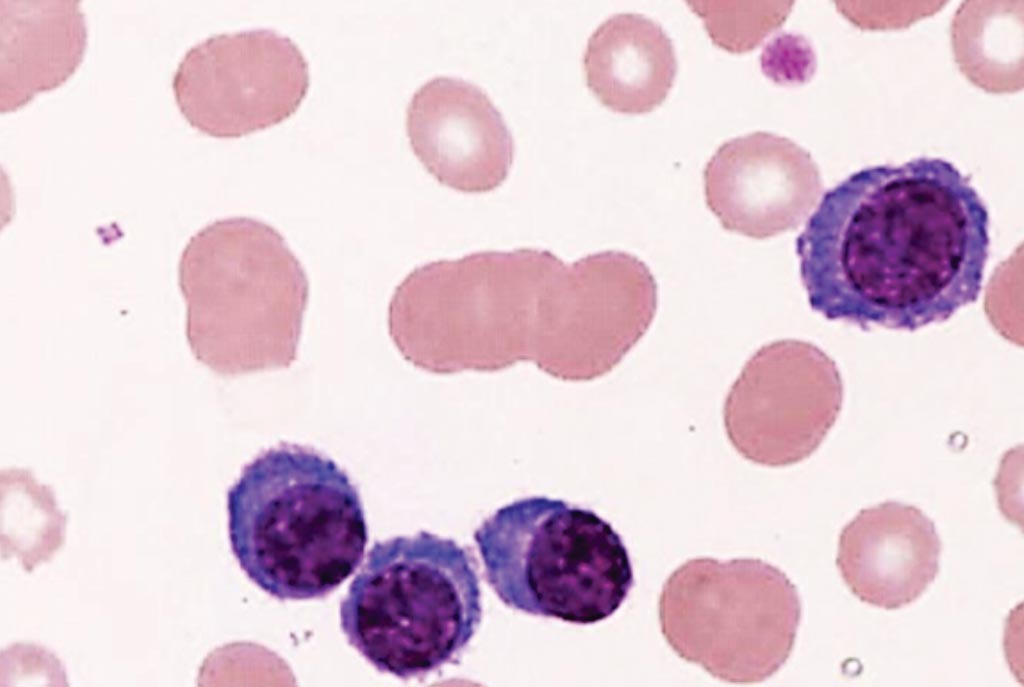
Image: A peripheral blood film from a patient with plasma cell leukemia showing four plasma cells and rouleaux formation of erythrocytes (Photo courtesy of the Tsuyako Saito).
Hematologists at the Mayo Clinic (Rochester, MN, USA) included in their study 176 patients, for which the median age was 62 years and the majority of which were men (56%). Similar numbers of patients were in each group: 54 patients (31%) had less than 5% CPCs, 63 patients (36%) had between 5% and 19% CPCs, and 59 patients (34%) had 20% or greater CPCs. The percentage of CPCs was derived from the conventional white blood cell differential count, which consists of a microscopic evaluation of Wright-Giemsa-stained peripheral blood smears. Cytogenetic information was collected from patients who underwent fluorescence in-situ hybridization (FISH) evaluation within six months of diagnosis and used to classify these patients as standard risk.
The median overall survival (OS) was 1.4 years for less than 5% CPCs, 1.1 years for between 5% and 19% CPCs, and 1.1 years for 20% or greater CPCs. Due to the similarity in the latter two groups, the study authors re-stratified patients as having either less than 5% or at least 5% CPCs. The median OS for patients with less than 5% CPCs (54 individuals) was 1.4 years and 1.1 years for patients with at least 5% CPCs. To further explore the relationship between CPC count and survival, the study authors compared these 122 patients with 5% or greater CPCs to a cohort of patients who received a diagnosis of multiple myeloma between 1971 and 2016 but did not have detectable CPCs (9,724 individuals).
Patients with 5% or greater CPCs lived a median of three years less than patients with no detectable CPCs (1.1 versus 4.4 years). When the time period of diagnosis was restricted to 2001 or later, the survival difference became even more pronounced. Patients with 5% or greater CPCs lived a median of six years fewer than patients with standard-risk multiple myeloma (1.4 years versus 7.5 years, respectively).
Wilson Gonsalves, MD, an oncologist and a co-author of the study, said, “The current definition of plasma cell leukemia was too restrictive. Many more myeloma patients exist with 5% to 19% circulating plasma cells that behave phenotypically similar to those who have 20% or more circulating plasma cells.” The authors proposed that PCL should be defined as the presence of ≥ 5% CPCs on a conventional peripheral blood smear in patients who meet diagnostic criteria for MM. PCL is a clinically and biologically distinct entity from MM, and specifically high-risk MM, and carries a worse prognosis. The study was published on November 15, 2018, in the Blood Cancer Journal.
Related Links:
Mayo Clinic













