CRISPR Illuminates Pediatric Bone Marrow Failure Syndrome
By LabMedica International staff writers
Posted on 10 Aug 2017
Children with dyskeratosis congenita experience progressive bone marrow failure, eventually losing the ability to make red blood cells, white blood cells and platelets and they are also at high risk of leukemia.Posted on 10 Aug 2017
Patients with dyskeratosis congenita (DC) usually come to clinical attention during childhood and present with a triad of oral leukoplakia, reticular skin pigmentation, and nail dystrophy. While the severity of DC varies across patients, more than 85% of afflicted individuals have cytopenias in one or more lineages in late childhood, and more than 95% will develop pancytopenia by adulthood.
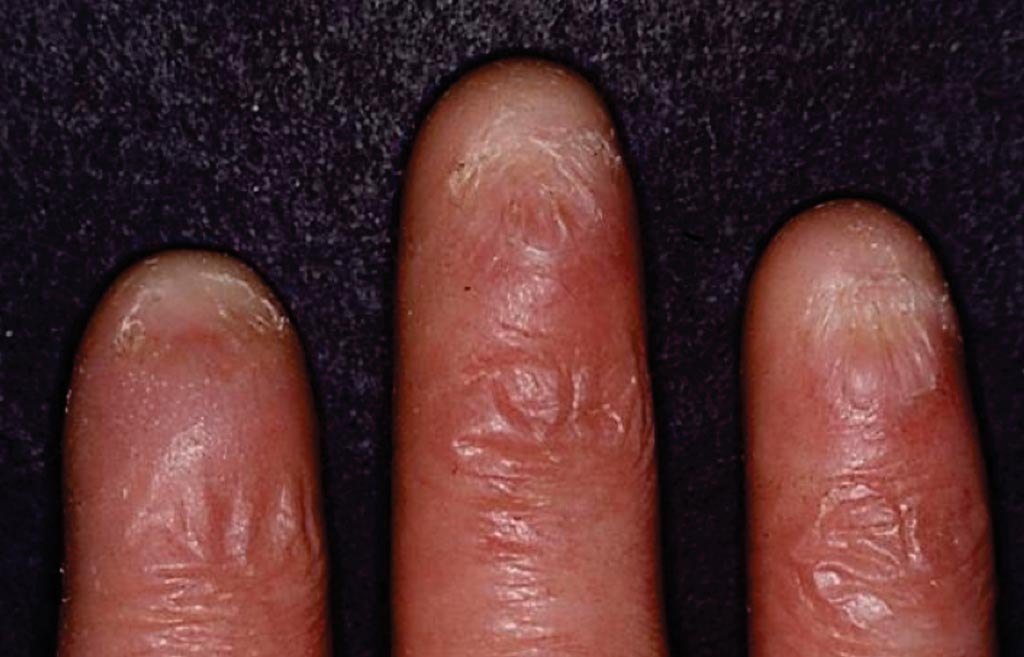
Image: Dyskeratosis congenita manifests as nail dystrophy where nail findings include ridging and longitudinal splitting. The nails may become thinned and atrophic leading to pterygium formation or absent nails. The changes in the fingernails and toenails may worsen over time (Photo courtesy of Dr. Maurice van Steensel).
Scientists at the Washington University in St. Louis (MO, USA) and their colleagues used the editing technology known as clustered regularly interspaced short palindromic repeats (CRISPR), which are segments of prokaryotic DNA containing short, repetitive base sequences. The Cas9 endonuclease is a four-component system that includes two small RNA molecules named CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA).
Although there has been extensive biochemical characterization of telomere maintenance mechanisms and their role in maintaining genomic integrity, the connection between telomere dysfunction and the specific clinical phenotypes of bone marrow failure (BMF) in DC patients remains poorly understood. Patient samples are rare and cannot address the effect of telomere deficiency on the genesis of tissue failure that occurs during hematopoietic development. The team characterized the primitive and definitive hematopoietic development of isogenic human embryonic stem cells (hESCs) carrying disease-associated mutations in the telomerase components: telomerase reverse transcriptase (TERT) and dyskerin pseudouridine synthase 1 (DKC1), two of the most commonly mutated genes in DC.
With this model, the scientists further showed how the telomere defect leads to the gradual loss of blood cell formation from human embryonic stem cells and, importantly, how blocking the downstream effects of the defect can reverse this loss, leading to normal production of blood cells. Blocking this signaling pathway did not lengthen telomeres or stop their shortening, but allowed the manufacturing of different types of blood cells to continue. Interestingly, they implicated high levels of a protein called p53 as one of the signals that leads to the drop in adult-like blood cell formation. The p53 protein is usually considered protective of DNA.
Luis F.Z. Batista, PhD, an assistant professor of medicine and senior author of the study said, “P53 is thought of as a guardian of the genome. Mutations that disable p53 are associated with different types of cancer. Because of this we would not consider directly trying to block p53 in these patients. But what this study provides is proof-of-concept that this pathway is involved in this response. So we now are looking for ways to block the pathway further downstream without necessarily blocking p53 directly.” The study was published on July 27, 2017, in the journal Stem Cell Reports.
Related Links:
Washington University in St. Louis














