Genetic Test Predicts Adverse Reaction To Toxic Goiter Treatment
By LabMedica International staff writers
Posted on 26 May 2016
Agranulocytosis is an acute condition involving a severe and dangerous leukopenia or lowered white blood cell count and drug-induced agranulocytosis is a potentially life-threatening adverse reaction, and adverse drugs reactions are a leading cause of admission to hospital.Posted on 26 May 2016
Genetic variation is believed to contribute to a majority of serious immune-mediated adverse drug reactions and gene variants have been found that predict the risk of a serious adverse reaction to drugs used for the treatment of hyperthyroidism, which is due to due to excessive production of thyroid hormone by the thyroid gland.
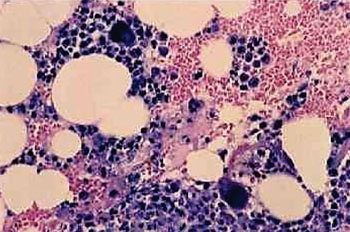
Image: A photomicrograph of a bone marrow aspiration biopsy showing drug-induced agranulocytosis (Photo courtesy of NMH).
A large number of international scientists and doctors led by those at Uppsala University (Sweden) are developing tests to predict patients at high risk of suffering side effects so that they can be offered other treatment. In the long run, this could lead to safer and more individualized treatment. They performed a genome-wide association study (GWAS) on 234 European adults with any non-chemotherapy drug-induced agranulocytosis where the absolute neutrophil count was equal to or less than 0·5 × 109/L (less than 500/μL) and 5,170 population controls. There were 39 of the 234 patients who had agranulocytosis that was induced by antithyroid drugs. After imputation and human leukocyte antigen (HLA) allele prediction, 9,380,034 single nucleotide polymorphisms (SNPs) and 180 HLA alleles were tested for association.
Agranulocytosis induced by non-chemotherapy drugs in general was significantly associated with the HLA region on chromosome 6. Drug-specific analysis showed that the association with HLA-B*27:05 was largely driven by cases induced by antithyroid drugs. In heterozygous carriers three specific SNPs predicted probability of antithyroid drug-induced agranulocytosis was about 30%. To avoid one case of agranulocytosis based on the possible risk reduction if all three SNPs are genotyped and carriers are treated or monitored differently from non-carriers, roughly 238 patients would need to be genotyped.
Mia Wadelius, MD, PhD, a senior physician and lead author of the study said, “'Some patients treated with medication for hyperthyroidism, such as thiamazole (methimazole), carbimazole or propylthiouracil, react with agranulocytosis which is a lack of white blood cells that suppresses the immune system. We have shown that certain immune genes increase the risk of agranulocytosis by 750 times in Europeans. This gives us an opportunity to individualize treatment using genetic testing and thus avoid an unnecessary adverse reaction.” The study was published on May 3, 2016, in the journal Lancet Diabetes & Endocrinology.
Related Links:
Uppsala University













