Advanced Next-Generation Sequencing Assay Kit Now Available for the Diagnosis of Leukodystrophy
By LabMedica International staff writers
Posted on 04 Aug 2015
An advanced next-generation sequencing (NGS) assay kit is now available to augment the use of MRI scans to diagnose the class of brain disorders known as leukodystrophies. Posted on 04 Aug 2015
Leukodystrophies are genetic disorders affecting the white matter of the central nervous system (CNS) with or without peripheral nervous system involvement. Although features vary, all have white matter abnormalities and most have motor deficits that dominate the clinical picture. The diagnosis of leukodystrophies are challenging due to the overall heterogeneous phenotypes, although an early diagnosis is critical because there are treatments available. The incidence rate of leukodystrophies is as high as one in 7,663 so they are relatively common for rare disorders.
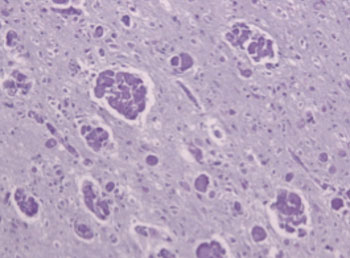
Image: Globoid cell leukodystrophy: Mulinucleated macrophages (\"globoid cells\") and loss of myelinated fibers in a case of Krabbe\'s leukodystrophy (Photo courtesy of Wikimedia Commons).
When damage occurs to white matter, immune responses can lead to inflammation in the CNS, along with loss of myelin. The degeneration of white matter can be seen in a MRI and is used to diagnose leukodystrophy. Leukodystrophy is characterized by specific symptoms including decreased motor function, muscle rigidity, and eventually degeneration of sight and hearing. While the disease is fatal, the age of onset is a key factor as infants are given a lifespan of two to eight years (sometimes longer), while adults typically live more than a decade after onset.
The biomedical company Transgenomic, Inc. (Omaha, NB, USA) recently released its Leukodystrophy NGS Panel for the diagnosis of leukodystrophy. Transgenomic, Inc. is a global biotechnology company advancing personalized medicine in cardiology, oncology, and inherited diseases through advanced diagnostic technologies. Transgenomic also provides specialized clinical and research services to biopharmaceutical companies developing targeted therapies and sells equipment, reagents and other consumables for applications in molecular testing and cytogenetics.
The Leukodystrophy NGS Panel analyzes 137 genes known to cause leukodystrophies and genetic leukoencephalopathies. The genes within this panel were recommended by the Global Leukodystrophy Initiative (Washington DC, USA) consensus for the diagnosis of these conditions. This approach to genetic testing can correctly identify the source of the patient’s disorder quickly. This allows for improved patient management decisions, including avoiding unnecessary interventions and diagnostic testing, and identifying treatable conditions.
The Leukodystrophy NGS Panel has sensitivity and specificity of greater than 99% while the average depth is 124X. It covers all coding exons and 10 base pairs of flanking intronic sequences for all targeted genes. All reported variants are confirmed with Sanger sequencing to ensure a high level of analytical specificity. Parental testing is offered to further explore the pathogenicity of all variants of unknown significance (VUS) identified in the patient that may be sufficient to cause disease.
“Leukodystrophy comprises a group of devastating genetic diseases that have been difficult to diagnose and almost impossible to treat,” said Paul Kinnon, president and CEO of Transgenomic. “We accordingly are very pleased to launch our Leukodystrophy NGS Panel, the most comprehensive genetic test for these conditions available today. By enabling clinicians to pinpoint the genetic source of the disorder earlier in the disease, we hope the Leukodystrophy NGS Panel will enable better disease management and facilitate the development of more effective therapies using new technologies, such as stem cells or gene editing techniques. This new test is another example of our commitment to harnessing advanced genetic tools to improve the lives of patients and families.”
Related Links:
Transgenomic, Inc.
Global Leukodystrophy Initiative