Dry-Reagent-Based Molecular Assay Facilitates Diagnosis of Buruli Ulcer
By LabMedica International staff writers
Posted on 20 Apr 2015
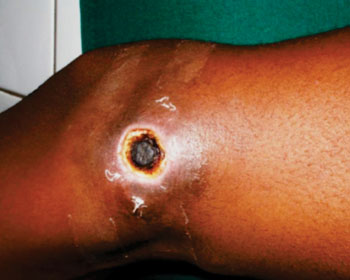
Buruli ulcer is a neglected tropical disease caused by Mycobacterium ulcerans causing a skin disease which is the third most common mycobacterial disease and its rapid diagnosis and treatment are essential.Posted on 20 Apr 2015
Polymerase chain reaction (PCR) is considered to be the most sensitive method for the laboratory confirmation of Buruli ulcer, but this technique remains expensive and involves reagents unsuitable for use in tropical countries with poor storage conditions, hindering the development of reliable quantitative PCR (qPCR) diagnosis.
Scientists from various French institutions including the University of Angers (France) compared the efficiency of three different dry qPCR mixes, lyophilized with various concentrations of cryoprotectants, with that of a freshly prepared mixture, for the detection of a standard range of M. ulcerans DNA concentrations. They evaluated the heat resistance of the dry mixes, comparing them with the fresh mix after heating. They also evaluated one of the dry mixes in field conditions, by analyzing 93 specimens from patients with suspected Buruli ulcers.
For validation of the dry mix in field conditions, the selected dry mix was evaluated on 48 swabs, 27 tissue samples, and 18 fine-needle aspirates (FNAs) from 93 patients with suspected Buruli ulcer. DNA amplification and detection were performed with a Chromo4 thermal cycler (Bio-Rad; Hercules, CA, USA). The dry mix was highly resistant to heat, of similar sensitivity and efficiency to the fresh mix and easier to use than the fresh mix. The investigators found 55 specimens were positive for M. ulcerans DNA and 38 were negative.
The authors concluded that dry qPCR mixes are suitable for use in the diagnosis of M. ulcerans infection in endemic countries. The user-friendly format of this mix makes it possible for untrained staff to perform diagnostic tests with a limited risk of contamination. The possibility of using this mix in either vial or strip form provides considerable flexibility for the management of small or large amounts of sample. The dry-mix qPCR could be used as a reliable tool for the diagnosis of Buruli ulcer in the field. The study was published on April 1, 2015, in the journal Public Library of Science Neglected Tropical Diseases.
Related Links:
University of Angers
Bio-Rad