DNA Blood Test Evaluated for Colorectal Cancer Detection
By LabMedica International staff writers
Posted on 11 Jun 2014
Tumor-derived aberrant DNA can be detectable in blood and blood tests have the potential to improve screening for colorectal cancer.Posted on 11 Jun 2014
A new blood test for bowel cancer based on two genes that are frequently hyper-methylated in colorectal adenoma and cancer (CRC) tissues, has been assessed for accuracy.
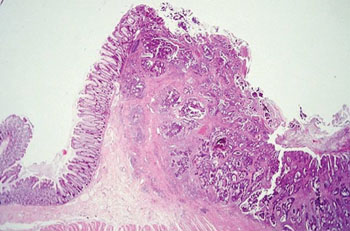
Image: Histopathology of a colon with adenocarcinoma showing chaotic appearance of glandular pattern (Photo courtesy of Johns Hopkins University).
Scientists at Flinders University (Adelaide, SA, Australia) working with their Dutch colleagues enrolled 2,187 volunteers with a mean age of 62 years, 52% of which were male. The patients were scheduled for colonoscopy for any reason, or for resection of colonic neoplasia, and were included if they provided sufficient plasma. The test was run successfully on 2,139 plasma specimens including 30 sample pairs taken pre- and post-surgical removal of neoplastic lesions.
Circulating cell-free DNA was extracted from plasma samples and bisulphite converted. Levels of recovered DNA and methylated Branched Chain Amino-Acid Transaminase 1, Cytosolic (BCAT1) and IKAROS Family Zinc Finger 1 (IKZF1) DNA were simultaneously determined using multiplexed quantitative polymerase chain reactions (qPCR) (Clinical Genomics; Sydney, NSW, Australia). A plasma sample was defined as positive if amplified target sequence signal above background fluorescence was present in any of three replicates in the BCAT1 or IKZF1 qPCR assay. Sensitivity and specificity for neoplasia were estimated as the primary outcome measures.
The two-gene blood test, BCAT1 and/or IKZF1 positive, successfully identified 85/130 (65.4%) of CRC cases. The successful detection rate increased to 73% for cancers that are Stage II or higher. The 12 BCAT1/IKZF1 methylation-positive CRC subjects with paired pre- and post-surgery plasma showed reduction in methylation signal after surgery, with complete disappearance of signal in 10 subjects. The presence of cancer-derived methylated DNA markers in plasma correlated with depth of tumor invasion and given the high rate of marker disappearance after cancer resection, the panel might also be useful to monitor tumor recurrence.
Graeme P. Young, MD, a professor of Gastroenterology and lead author of the study said, “A blood test is likely to overcome some of the barriers to screening with fecal tests. It might prove to be acceptable to those failing to participate in screening using established methods, which at the moment are primarily based around fecal tests.” The study was published on April 12, 2014, in the journal Gastrointestinal Endoscopy.
Related Links:
Flinders University
Clinical Genomics