Gold Nanoparticle-Based Test Better for Prostate Cancer Diagnosis Than PSA
By LabMedica International staff writers
Posted on 14 Apr 2015
A rapid and simple gold nanoparticle-based blood test proved better at diagnosing prostate cancer than did the commonly used PSA (prostate-specific antigen) assay.Posted on 14 Apr 2015
Investigators at the University of Central Florida (Orlando, USA) based their NanoDLSay test on the ability of gold nanoparticles to bind certain proteins from the serum and to increase in size and change their light scattering properties.
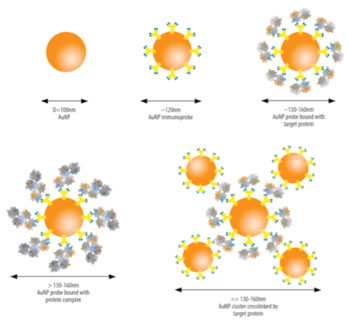
Image: Illustration of the NanoDLSay light scattering assay for detecting target analytes by measuring nanoparticle size change (Photo courtesy of Nano Discovery Inc.).
When citrate ligands-capped gold nanoparticles are mixed with blood sera, a protein corona is formed on the nanoparticle surface due to the adsorption of various proteins in the blood to the nanoparticles. Using a two-step gold nanoparticle-enabled dynamic light scattering assay, the investigators discovered that the amount of human immunoglobulin G (IgG) in the gold nanoparticle protein corona was increased in prostate cancer patients compared to healthy controls. Two pilot studies conducted on blood serum samples collected at Florida Hospital (USA) and obtained from the Prostate Cancer Biorespository Network revealed that the test had 90%–95% specificity and 50% sensitivity in detecting early stage prostate cancer, representing a significant improvement over the current PSA test.
The investigators postulated that the increased amount of human IgG found in the protein corona was associated with the autoantibodies produced in cancer patients as part of the immune system's defense against the tumor. Proteomic analysis of the nanoparticle protein corona revealed molecular profile differences between serum samples from cancer patients and healthy individuals. Autoantibodies and natural antibodies produced in cancer patients in response to tumorigenesis have been found and detected in the blood of many cancer types. Thus, the test may be applicable for early detection and risk assessment of a broad spectrum of cancer types.
"What is different and unique about our technique is it is a very simple process, and the material required for the test is less than one USD," said senior author Dr. Qun Huo, professor of chemistry at the University of Central Florida. "And because it is low-cost, we are hoping most people can have this test in their doctor's office. If we can catch this cancer in its early stages, the impact is going to be big."
The investigators have founded the biomedical company Nano Discovery Inc. (Orlando, FL, USA) to commercialize the NanoDLSay diagnostic test for prostate cancer.
The report was published in the March 10, 2015, online edition of the journal ACS Applied Materials & Interfaces.
Related Links:
University of Central Florida
Nano Discovery Inc.











.jpg)