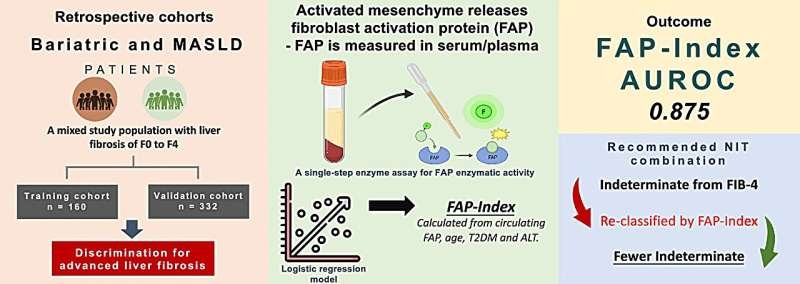
High-Sensitivity Cardiac Troponin T Identifies Myocardial Injury with COVID-19
By LabMedica International staff writers
Posted on 01 Sep 2021
Cardiac troponin (cTn) is the preferred biomarker for the detection of myocardial injury (MI), which is defined when there is at least one cTn concentration above the 99th percentile upper-reference limit (URL) of a healthy reference cohort.Posted on 01 Sep 2021
While pulmonary complications are frequent with COVID-19, studies suggest that myocardial injury is common, in particular among those with chronic cardiovascular conditions and more severe COVID-19 presentations, and that its presence and magnitude is associated with worse outcomes.
Image: The Automated Elecsys Troponin T Gen 5 STAT assay facilitates the diagnosis of myocardial infarction, also in patients with COVID-19 (Photo courtesy of Roche Diagnostics)
Cardiologist and a large team of medical scientists at the Mayo Clinic (Rochester, MN, USA) conducted a multicenter, retrospective, observational, US-based study of COVID-19 patients undergoing high-sensitivity cardiac troponin T (hs-cTnT). The final study cohort included 367 COVID-19 patients in whom at least one hs-cTnT was obtained, amongst which 46% were identified to have myocardial injury based on hs-cTnT concentrations above the sex-specific 99th percentiles. The mean ± SD age of the cohort was 61 ±17 years and most patients presented through the Emergency Department (83%).
The hs-cTnT was measured with the Elecsys Troponin T Gen 5 STAT (Roche Diagnostics, Indianapolis, IN, USA). The lowest reportable clinical value is the <6 ng/L limit of quantification (LoQ). Sex-specific 99th percentiles URLs of 10 ng/L for women and 15 ng/L for men were used. Patients with suspected MI are evaluated using a 0/2 hour hs-cTnT protocol that uses sex-specific 99th percentile URLs to rule-in and rule-out myocardial injury and an absolute delta (serial change) of ≥10 ng/L to identify patients with acute injury. MI is diagnosed when there is objective evidence of myocardial ischemia.
The team reported that among 367 COVID-19 patients undergoing hs-cTnT, myocardial injury was identified in 46%. They had a higher risk for mortality (20% versus 12%), unadjusted hazard ratio (HR) of 4.44 and major adverse events (35% versus 11%). The hs-cTnT results were independent predictors of major adverse events. Most (95%) increases were due to myocardial injury, with 5% classified as type 1 or 2 myocardial infarction. A single hs-cTnT <6 ng/L identified 26% of patients without mortality, with a 94.9% (negative predictive value and 93.1% sensitivity for major adverse events in those presenting to the ED.
The authors concluded that myocardial injury is frequent and prognostic in COVID-19. While most hs-cTnT increases are modest and due to myocardial injury, they have important prognostic implications. A single hs-cTnT <6 ng/L at presentation may facilitate the identification of patients with a favorable prognosis. The study was published in the August 2021 issue of the journal Clinical Chemistry.
Related Links:
Mayo Clinic
Roche Diagnostics