CSF Conversion Assay Identifies Lewy Body Disease Cognitive Impairment
By LabMedica International staff writers
Posted on 08 Aug 2021
Lewy body dementia, also known as dementia with Lewy bodies, is the second most common type of progressive dementia after Alzheimer's disease. Protein deposits, called Lewy bodies, develop in nerve cells in the brain regions involved in thinking, memory and movement (motor control).Posted on 08 Aug 2021
Real-time quaking-induced conversion (RT-QuIC) is a highly sensitive assay originally used for prion detection. The "quaking" in the name of the technique refers to the fact that samples in the RT-QuIC assay are literally subjected to shaking. This action breaks apart aggregates of protein that are then further incubated, amplifying the amount of protein to detectable levels.
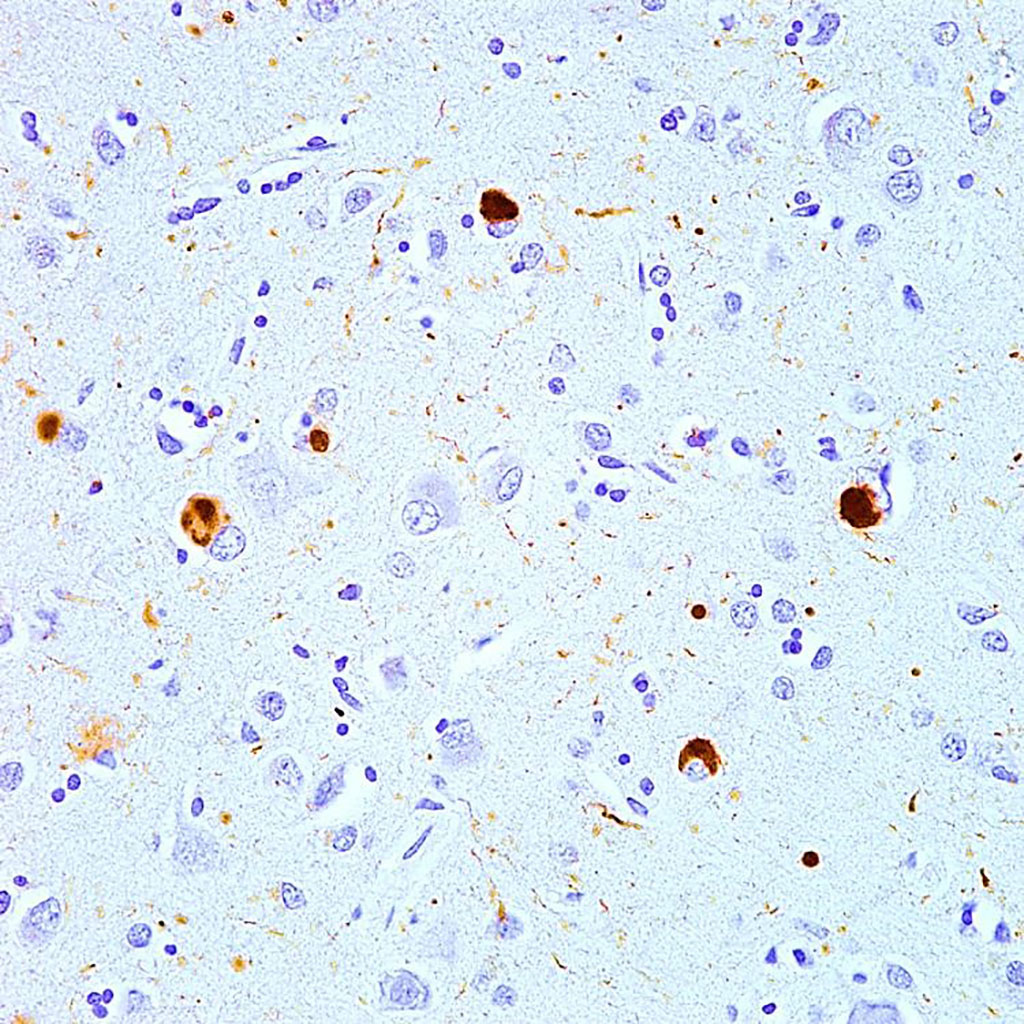
Photomicrograph shows brown-immunostained alpha-synuclein in Lewy bodies (large clumps) and Lewy neurites (thread-like structures) in the neocortical tissue of a person who died with Lewy body disease (Photo courtesy of Movalley)
Neurology Scientists at the Institute of Neurological Sciences of Bologna (Bologna, Italy) investigated whether the cerebrospinal fluid (CSF) α-synuclein (α-syn) real-time quaking-induced conversion (RT-QuIC) assay accurately identifies patients with mild cognitive impairment due to probable Lewy body disease (MCI-LB).
The team applied α-syn RT-QuIC to 289 CSF samples obtained from two independent cohorts, including 81 patients with probable MCI-LB (70.7± 6.6 years, 13.6% female, Mini-Mental State Examination (MMSE) 26.1 ± 2.4), 120 with probable MCI-AD (68.6 ± 7.4 years, 45.8% female, MMSE 25.5 ± 2.8), and 30 with unspecified MCI (65.4 ± 9.3 years, 30.0% female, MMSE 27.0 ± 3 .0). Fifty-eight individuals with no cognitive decline or evidence of neurodegenerative disease and 121 individuals lacking brain α-syn deposits at the neuropathological examination were used as controls.
The investigators reported that RT-QuIC identified MCI-LB patients against cognitively unimpaired controls with 95% sensitivity, 97% specificity, and 96% accuracy, and showed 98% specificity in neuropathological controls. The accuracy of the test for MCI-LB was consistent between the two cohorts (97.3% versus 93.7%). Thirteen percent of MCI-AD patients also had a positive test; of note, 44% of them developed one core or supportive clinical feature of dementia with Lewy bodies (DLB) at follow-up, suggesting an underlying LB co-pathology.
The scientists noted that taken together, these findings are in line with preliminary results obtained in patients with isolated REM sleep behavior disorder, pure autonomic failure and those with incidental Lewy body dementia at post-mortem examination, demonstrating that patients with Lewy body dementia harbor significant alpha-synuclein seeding activity early in the course of the disease, irrespective of clinical presentation.
The authors concluded that their findings indicated that CSF α-syn RT-QuIC is a robust biomarker for prodromal DLB and accurately identified dementia with Lewy bodies in patients at the prodromal clinical stage and demonstrated high specificity in a large cohort of individuals examined neuropathologically. The study was published on July 1, 2021 in the journal Neurology.
Related Links:
Institute of Neurological Sciences of Bologna