Ovarian Cancer Protein Contributes to Alzheimer`s Disease Neurodegeneration
By LabMedica International staff writers
Posted on 30 Jan 2020
Alzheimer's disease is a complex neurological disorder with pathological hallmarks of hyperamyloidosis (senile plaques), neurofibrillary tangles containing hyperphosphorylated tau, and extensive neurodegeneration in the brain.Posted on 30 Jan 2020
Alzheimer's disease (AD) pathogenesis remains elusive and no effective therapy is available. Neurodegeneration, including synaptic damage and neuronal loss, forms the basis of dementia in AD, and certain brain regions are more vulnerable during disease progression.
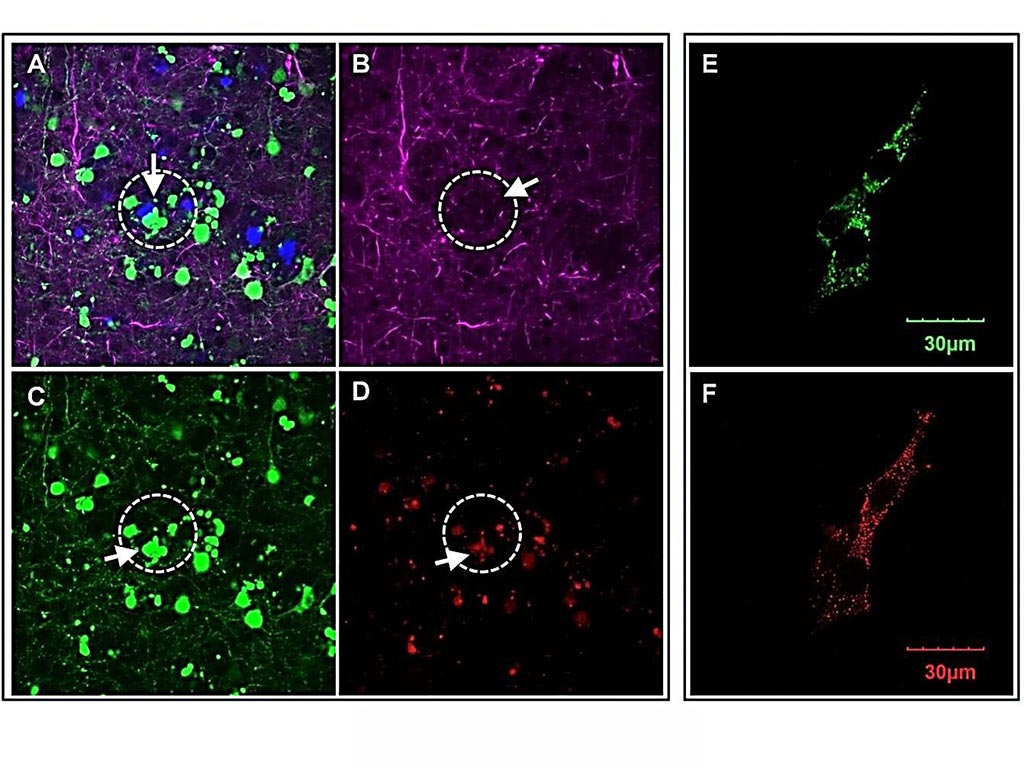
Image: In the brain of mice with Alzheimer\'s, areas near amyloid plaques (A) appear with fewer neural networks (B), dying neurons (C) and higher OCIAD1 (D). In cultured neuronal cells, the OCIAD1 proteins (E) appear in the mitochondria (F) (Photo courtesy of Houston Methodist Research Institute).
Scientists at the Houston Methodist Research Institute (Houston, TX, USA) and their colleagues reported on a new role of ovarian cancer immune-reactive antigen domain containing 1 (OCIAD1). Originally discovered for its effect on ovarian cancer metastasis and stem cell metabolisms, the group found the OCIAD1 protein in human brain cells, and determined it impairs neurons and damages synapses in the brain, contributing to neurodegeneration in Alzheimer's disease.
The investigators culled through archived bioinformatics data of brain tissue from deceased Alzheimer's patients, as well as mouse models by blending computational methods with laboratory studies. They determined that OCIAD1 plays a role in the disease's progressive neurodegeneration by impairing mitochondria function. Known as the powerhouse of cells, damage to mitochondria results in the trickle-down cell death effect in the brain leading to neuron damage.
Higher levels of OCIAD1, found in vulnerable brain areas and dystrophic neurites, were correlated with disease severity. Multiple early AD pathological events, particularly Aβ/GSK-3β signaling, elevated OCIAD1, which in turn interacts with BCL-2 to impair mitochondrial function and facilitates mitochondria-associated neuronal injury. Notably, elevated OCIAD1 by Aβ increases cell susceptibility to other AD pathological challenges.
Xuping Li, PhD, an instructor of Neurodegeneration in Oncology and co-corresponding author, said, “We applied a system biology strategy to see if we could find a different mechanism of neurodegeneration in Alzheimer's disease. We identified OCIAD1 as a new neurodegeneration-relevant factor, predicted its function, and demonstrated it mediates the long-term impact of amyloid beta on cells and synaptic damages by impairing mitochondria function.” The study was published on January 12, 2020 in the journal EBioMedicine.
Related Links:
Houston Methodist Research Institute














