C-Reactive Protein Point-Of-Care Test Evaluated
By LabMedica International staff writers
Posted on 27 Aug 2019
C-reactive protein (CRP) is a key mediator of the acute-phase response, with blood levels of CRP increasing rapidly after an inflammatory stimulus. Therefore, changes in serum levels of CRP are a clinically useful marker of infection, inflammation, and tissue injury.Posted on 27 Aug 2019
CRP testing in the primary setting as in point-of-care (POC) can help reduce diagnostic uncertainty by differentiating between bacterial and viral infections and has been shown to be cost-effective for reducing inappropriate antibiotic prescriptions. However, despite the availability of POC devices, POC CRP testing has not yet been widely adopted in primary care clinics.
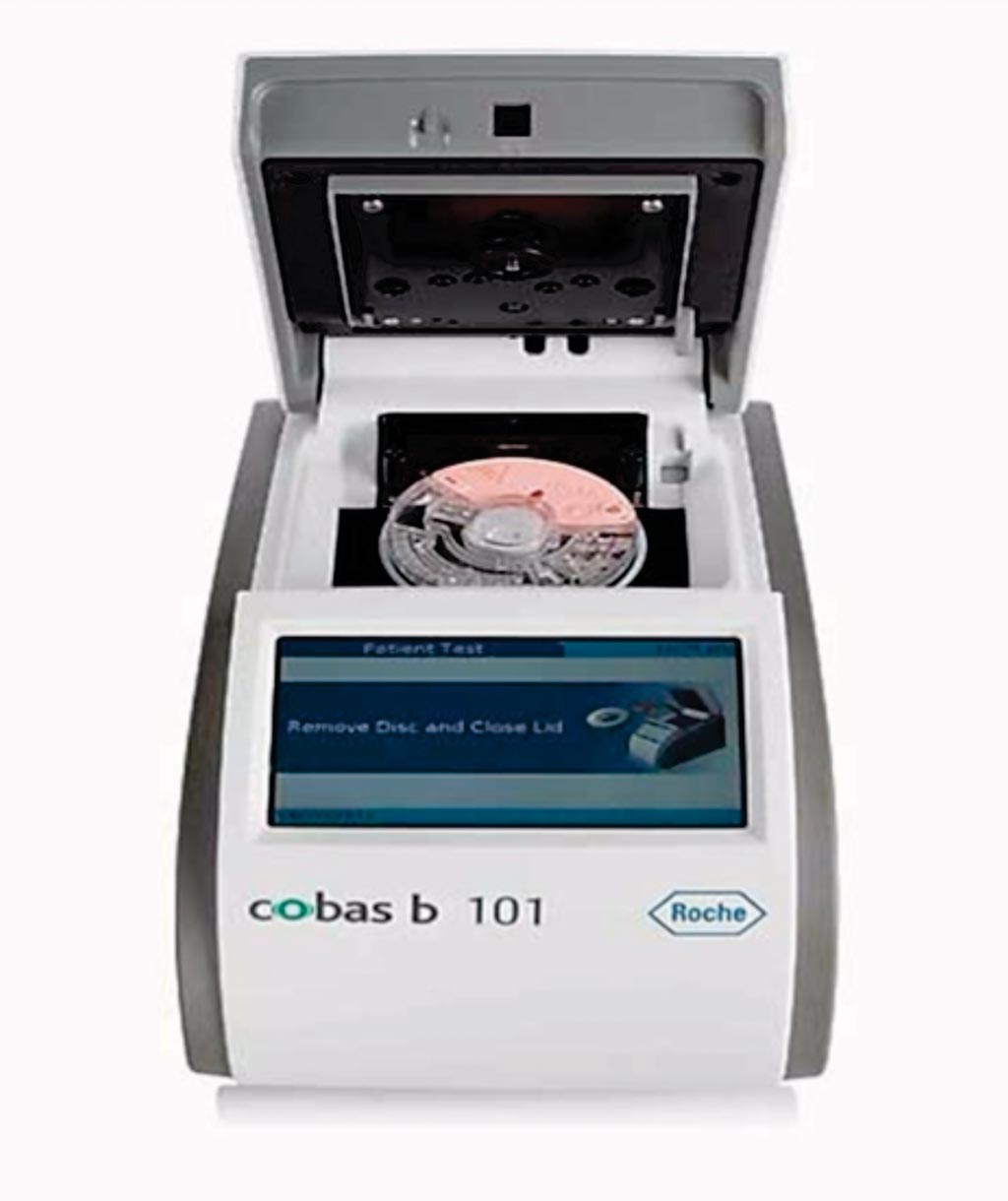
Image: The cobas b 101 POC system; an in vitro diagnostic (IVD) test system offering C-reactive protein, HbA1c and a complete lipid profile (CHOL, HDL, LDL, TG) on one device at the point of care (Photo courtesy of Roche Diagnostics).
Clinical laboratory scientists at the Catharina Hospital and Technical University Eindhoven (Eindhoven, The Netherland) and their colleagues evaluated a POC CPR test at three sites and one reference laboratory. Within-run (repeatability), within-laboratory (intermediate precision), and between-laboratory precision (reproducibility) were assessed. Method comparison and matrix/lot-to-lot comparison studies were conducted using prospectively collected blood samples from 217 adults (apparently healthy or with clinically relevant conditions).
The investigators compared the analytical performance of the cobas b 101 POC system, which provides glycated hemoglobin (HbA1c) and lipid panel tests (measurement of cholesterol, triglyceride, and high-density lipoprotein; calculation of low-density lipoprotein) for managing diabetes and dyslipidemia at point-of-need. This method was compared with a reference test: CRPNX reagent on a Roche cobas c 501 module.
The scientists reported that clinically relevant CRP concentrations measured with the CRP Test showed good agreement with those measured by CRPNX reagent. Coefficients of variation (CV) for repeatability and intermediate precision ranged from 1.7%–4.0% and 1.9%–4.5%, respectively, for human serum pools containing CRP 4.7–350.7 mg/L; repeatability in clinical samples ranged from 1.6%–5.9% (3.3–360.3 mg/L). CVs for reproducibility ranged from 2.5%–4.0% (4.7–344.3 mg/L). CRP concentrations were comparable for capillary whole blood, serum, Li-heparin whole blood/plasma, K2 and K3 EDTA whole blood/plasma. The overall mean usability score was 4.18/5 and the error rate across 9,378 tests was 1.00%.
The authors concluded that their findings indicate that healthcare professionals can obtain precise and reproducible CRP values with the cobas POC CRP Test that show very good correlation with laboratory measurements. Importantly, operators considered the system convenient for use in the POC environment. The study was published in the September 2019 issue of the journal Clinical Biochemistry.
Related Links:
Catharina Hospital and Technical University Eindhoven













