Nanopatterned Microfluidic Chip Detects Cancer Markers
By LabMedica International staff writers
Posted on 13 Mar 2019
The performance of current microfluidic methods for exosome detection is constrained by boundary conditions, as well as fundamental limits to microscale mass transfer and interfacial exosome binding.Posted on 13 Mar 2019
Some types of cancer, such as ovarian cancer, tend to remain undetected until they are too advanced for treatment to be effective. Now, an innovative tool may be able to detect cancer easily, quickly, and in minuscule amounts of blood. A device, which is called a "3D-nanopatterned microfluidic chip," could successfully detect cancer markers in the tiniest drop of blood or in a component of the blood called plasma.
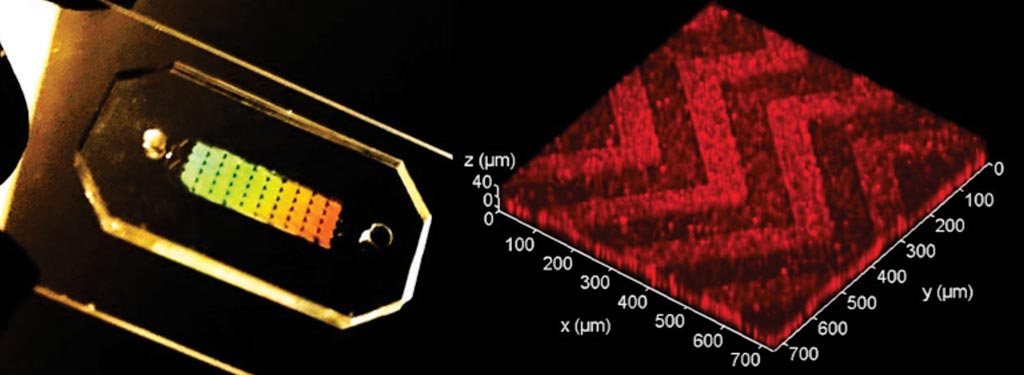
Image: The three-dimensional (3D) herringbone nanopatterned microfluidic chip that detects cancer faster (Photo courtesy of the University of Kansas).
Biochemists at the University of Kansas (Lawrence, KS, USA) and their colleagues have shown that a microfluidic chip designed with self-assembled three-dimensional herringbone nanopatterns can detect low levels of tumor-associated exosomes in plasma (10 exosomes μL−1, or approximately 200 vesicles per 20 μL of spiked sample) that would otherwise be undetectable by standard microfluidic systems for biosensing. The nanopatterns promote microscale mass transfer, increase surface area and probe density to enhance the efficiency and speed of exosome binding, and permit drainage of the boundary fluid to reduce near-surface hydrodynamic resistance, thus promoting particle–surface interactions for exosome binding.
The scientists used the device for the detection, in 2 μL plasma samples from 20 ovarian cancer patients and 10 age-matched controls, of exosome subpopulations expressing CD24, epithelial cell adhesion molecule and folate receptor alpha proteins. They suggest exosomal folate receptor alpha as a potential biomarker for early detection and progression monitoring of ovarian cancer. The nanolithography-free nanopatterned device should facilitate the use of liquid biopsies for cancer diagnosis.
Yong Zeng, PhD, an associate professor of chemistry and the senior author of the study, said, “Historically, people thought exosomes were like 'trash bags' that cells could use to dump unwanted cellular contents. But, in the past decade scientists realized they were quite useful for sending messages to recipient cells and communicating molecular information important in many biological functions. Basically, tumors send out exosomes packaging active molecules that mirror the biological features of the parental cells. While all cells produce exosomes, tumor cells are really active compared to normal cells.” The study was published on February 25, 2019, in the journal Nature Biomedical Engineering.
Related Links:
University of Kansas














