Salivary Urea Nitrogen Dipstick Detects Acute Kidney Disease
By LabMedica International staff writers
Posted on 02 Jan 2019
Early recognition of acute kidney injury (AKI) is an essential step in allowing timely treatment in efforts to improve patient outcomes from this potentially reversible condition.Posted on 02 Jan 2019
The diagnosis of AKI is highly dependent on the measurement of serum creatinine (SCr), which is almost universally available in high-income countries; however it is often not available in less privileged health care settings.
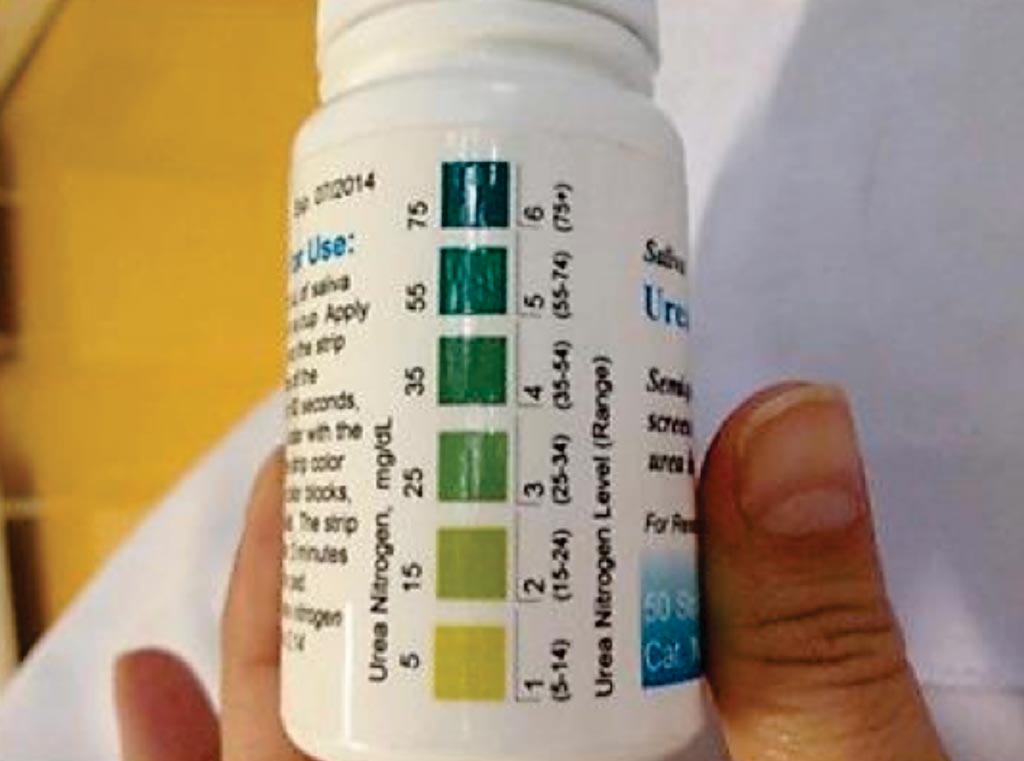
Image: A salivary urea nitrogen dipstick (Photo courtesy of University of Malawi College of Medicine).
Scientists collaborating with the University of Malawi College of Medicine (Blantyre, Malawi) enrolled 310 patients between September 21 and December 11, 2015, in a prospective observational study. Those include in the study were more than 20 weeks’ gestation or less than 6 weeks postpartum presenting with conditions predetermined as being at risk of leading to AKI. The mean age of the entire cohort was 25.9 ± 6.45 years.
Baseline clinical data were recorded, and screening for kidney disease (community-acquired) was undertaken with a SCr alongside simultaneous measurement of Salivary Urea Nitrogen (SUN) using a dipstick. SCr and urea were measured by the Jaffe and urease methods, respectively, using either a Flexor Junior Clinical Chemistry Analyzer or a Mindray Chemistry Analyzer BS-120.
The investigators reported that of the patients, 23 (7.6%) had AKI, stage 1 in 47.8%, most commonly due to preeclampsia/eclampsia. Mean presenting SCr values were 108.8 ± 21.8 μmol/L (1.23 ± 0.25 mg/dL), 118 ± 34.45 μmol/L (1.33 ± 0.39 mg/dl), and 136.1 ± 30.4 μmol/L (1.54 ± 0.34 mg/dL) in AKI stages 1 to 3 respectively. SUN greater than 14 mg/dL had a sensitivity of 12.82% and a specificity of 97.33% to detect acute kidney disease. The area under the receiver operating characteristic curve was 0.551. In patients with normal SUN on admission, perinatal mortality was 11.8%, and was 25.0% if SUN was > 14 mg/dL.
The authors concluded that in their study, measuring SUN using a dipstick was specific but insensitive when used to diagnose obstetric-related acute kidney disease in Malawi. Low salivary urea concentrations in this cohort, in combination with the reduced accuracy of the dipstick at lower levels of BUN, are the likely main drivers for the lack of sensitivity seen in the study. A modified SUN dipstick with increased sensitivity at the lower ranges of SUN is currently under development, with plans to test this in both pregnant and non-pregnant patients with kidney disease. The study was published in the January 2019 issue of the journal Kidney International Reports.
Related Links:
University of Malawi College of Medicine







 Analyzer.jpg)





